In a titration of HCl with NaOH, you read the buret to see how much base it
Question:
In a titration of HCl with NaOH, you read the buret to see how much base it took to neutralize the acid (turn the indicator pink). You do this titration to determine the molar concentration of the acid.
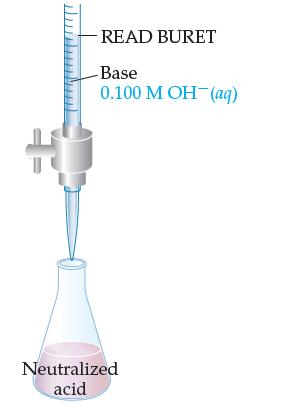
Why is it important to accurately know the amount (volume) of acid that you originally put into the flask?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





