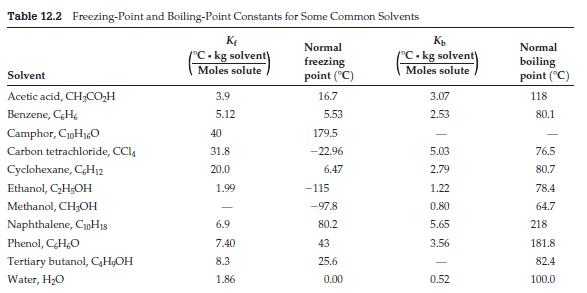
The solvent cyclohexane is in Table 12.2. The compound methyl lithium (CH 3 Li) is a solute
Question:
The solvent cyclohexane is in Table 12.2. The compound methyl lithium (CH3Li) is a solute that dissolves readily in cyclohexane. A simple way to think of methyl lithium is as an ionic compound made up of methyl anions (CH3–) and lithium cations (Li+). Dissolving 0.100 moles of it in 0.100 kg of cyclohexane yields a freezing point of 3.97 °C. Explain how this is consistent with the known structure of methyl lithium in solution, which is shown below
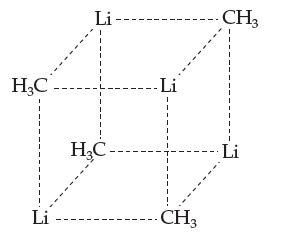
Table 12.2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





