In Reaction 15.7, water is shown acting as a base. How is water behaving in the following
Question:
In Reaction 15.7, water is shown acting as a base. How is water behaving in the following reaction?
![]()
Explain your answer.
Reaction 15.7
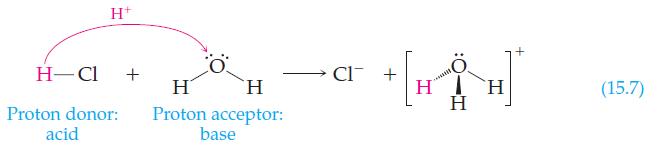
Transcribed Image Text:
NH₂ + H₂O → NH3 + OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Answer In the reaction NH 2 H 2 O NH 3 OH water is acting as an acid This can be understood through ...View the full answer

Answered By

Mamba Dedan
I am a computer scientist specializing in database management, OS, networking, and software development. I have a knack for database work, Operating systems, networking, and programming, I can give you the best solution on this without any hesitation. I have a knack in software development with key skills in UML diagrams, storyboarding, code development, software testing and implementation on several platforms.
4.90+
97+ Reviews
194+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
In Chapter 10, we will see that an acetylide ion (formed by treatment of acetylene with a strong base) can serve as a nucleophile in an S N 2 reaction: This reaction provides a useful method for...
-
In this part of the project you are to assume to have been hired to join a team serving as an internal financial analyst to THE COMPANY. Your client plans to invest in bonds and (or) stocks issued by...
-
It is important to be able to distinguish when an electron pair donor in a reaction is acting as a base or as a nucleophile. The following reactions are elementary reactions. In each case, determine...
-
Assume that the average talk time on an Apple iPhone is 20 hours and that this time follows the exponential probability distribution. What is the probability that a randomly selected iPhone will...
-
The financial statements for Highland Publications Corporation included the following selected information: Common stock $1,600,000 Retained earnings $900,000 Net income $1,000,000 Shares issued...
-
When trans-1-phenylpropene is treated with bromine, some syn addition is observed. Explain why the presence of a phenyl group causes a loss of stereospecificity. Br + En Br2 En Br Br Syn addition...
-
The transfer of funds by savers from checking accounts to saving accounts M1 and M2.
-
Sanlucas, Inc., provides home inspection services to its clients. The following is the company's trial balance dated June 1, current year. Sanlucas engaged in the following transactions in June. June...
-
When an investor buys an asset to be kept for at least a year, the net cost is the reduction in what the investor can spend without reducing other wealth. For investments in some types of retirement...
-
(a) How does a weak acid in a buffer eliminate added OH ions? To what does it convert these ions? What happens to the weak acid? (b) How does a weak base in a buffer eliminate added H 3 O + ions? To...
-
Draw three beakers as shown in WorkPatch 15.3, but change the labels to make them reflect the reaction mentioned in Practice Problem 15.9. Then list all species present in each beaker. Data from...
-
Following was the Balance Sheet of B Ltd. as on 31.3.2016: The company undertook following scheme of reconstruction: (a) Equity shares were to be reduced to shares of 50 each fully paid. (b)...
-
Part 1 - Financial Statement Analysis Income Statement Kirks Family Restaurant December 31, 2018 Sales 480,000 Interest revenue 15,000 Total Revenue 495,000 Cost of goods sold 200,000 Gross Margin...
-
Find the most general value of satisfying tan 0 = -3.
-
(i) Undercasting of the debit side of Bank column. 70 (ii) Cheques issued but not presented for payment till 01-01-2011. 1,450 (1,520) 2,179 Bank Balance as per Pass Book as on 1-1-2011. Different...
-
Determine the stiffness matrix K for the truss. Take A = 0.0015 m^2 and E = 200 GPa for each member. Please show the step-by-step solution. 5 410 9 3 5 7 7 8 A4 Tesol242 3 2 4 4 5 6 2 4 m 4 m 20 kN...
-
Probability Mr Pandazis Practice Questions for Test #1 Math 241 1. Define a sample space S for the following experiment. Toss a coin three times and record the outcome for each toss. 2. A card is...
-
On May 15, Helena Carpet Inc., a carpet wholesaler, issued for cash 750,000 shares of no-par common stock (with a stated value of $1.50) at $4, and on June 30, it issued for cash 17,500 shares of...
-
Classify each of the following activities as proper or prohibited under the various consumer statutes you have studied. a. Calling a hospital room to talk to a debtor who is a patient there. b....
-
The structural assembly supports the loading shown. Draw the moment diagrams for each of the beams. Take I = 100(10 6 ) mm 4 for the beams and A = 200 mm 2 for the tie rod. All members are made of...
-
The contilevered beam is supported at one end by a 1/2 in.-diameter suspender rod AC and fixed at the other end B. Determine the force in the rod due to a uniform loading of 4 k/ft. E = 29(10 3 ) ksi...
-
The beam AB has a moment of inertia I = 475 in 4 and rests on the smooth supports at its ends. A 0.75-in-diameter rod CD is welded to the center of the beam and to the fixed support at D. If the...
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


