Of the three solutions shown here, (a) Which is a weak electrolyte, which is a strong electrolyte,
Question:
Of the three solutions shown here,
(a) Which is a weak electrolyte, which is a strong electrolyte, and which is a nonelectrolyte? How can you tell?
(b) Which of these compounds is/are molecular? Which is/are ionic?
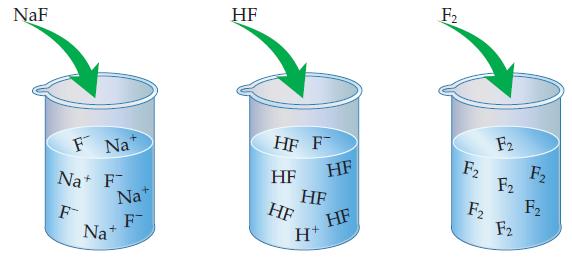
Transcribed Image Text:
NaF F Na Na+ F F Nat F Na+ HF HF F HF HF HF HF H+ HF F₂ F₂ F2 F2 F2 F2 F₂ F₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a NaF is a strong electrolyte because it dissociates completely HF i...View the full answer

Answered By

Shadrack Mulunga
I am a Biochemistry by profession. However, I have explored different fields of study. My quest to explore new fields has helped me gain new knowledge and skills in Business, clinical psychology, sociology, organizational behavior and general management, and Project Management. I count my expertise in Project management, in particular, creation of Work Break Down Structure (WBS) and use of Microsoft Project software as one of my greatest achievement in Freelancing industry. I have helped thousands of BSC and MSC students to complete their projects on time and cost-effectively using the MS Project tool. Generally, I find happiness in translating my knowledge and expertise to success of my clients. So far, i have helped thousands of students to not only complete their projects in time but also receive high grades in their respective courses. Quality and timely delivery are the two key aspects that define my work. All those who hired my services always come back for my service. If you hire my services today, you will surely return for more. Try me today!
5.00+
154+ Reviews
289+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
Part 1 a. Ammonia, NH 3 , is a weak electrolyte. It forms ions in solution by reacting with water molecules to form the ammonium ion and hydroxide ion. Write the balanced chemical reaction for this...
-
Three beakers contain clear, colorless liquids. One beaker contains pure water, another contains salt water, and another contains sugar water. How can you tell which beaker is which? (No tasting...
-
As the traffic manager for ABC Electronics, you have been charged with the task of reducing shipping costs for a fast selling cable product that is sold by the pound. You have a very satisfactory...
-
Preparing a Balance Sheet and Analyzing Some of Its Parts Exquisite Jewelers is developing its annual financial statements for 2012. The following amounts were correct at December 31, 2012: cash,...
-
When 1,3-dinitrobenzene is treated with nitric acid and sulfuric acid at elevated temperature, the product is 1,3,5-trinitrobenzene. Explain the regiochemical outcome of this reaction. In other...
-
What do you consider to be the benefits and drawbacks of shopping online for motor vehicles and other items?
-
A production line at V.J. Sugumarans machine shop has three stations. The first station can process a unit in 10 minutes. The second station has two identical machines, each of which can process a...
-
It has been determined that even though Switzerland has a lower interest rate, the U.S. would be taking a loss in the end because of inflation and exchange rates. What countries other than Brazil...
-
(a) Is the compound H 2 SO 4 molecular or ionic? (b) How did you decide on your answer to part (a)? (c) Based on your answer to part (a), would you call H 2 SO 4 an electrolyte or a nonelectrolyte?...
-
A substance is an electrolyte if: (a) It conducts electricity. (b) It can produce electricity. (c) An aqueous solution of it conducts electricity. (d) It is a neutral substance.
-
You must decide between job offers from CPM Construction and Fudd Associates. Both companies concentrate on sustainable commercial construction, have similar fringe benefits and offer comparable...
-
The ratio of CEO pay to that of an average employee increased over a period of 50 years from 24:1 to 275:1. Is this increasing gap ethically sound, in your opinion? Should CEO pay be limited in any...
-
Suppose you are considering buying a machine that costs $7,000. It will generate revenues of $1,500 for the next 3 years, and then $1,000 for the following 5 years. What is the payback period of this...
-
National Bakery Limited is the main supplier of a variety of baked products to customers in Kingston. The company currently makes 25,000,000 a variety of baked products annually which uses baking...
-
Q1. Discuss the financial goal of a business. Ensure to provide an example of the inherent ethical challenges associated with the financial goal and or the financial management process. Using the...
-
Q1. How can companies use social media to do sentiment analysis? Describe the process. Give an example of a company that uses sentiment analysis to enhance relationships with customers. Q2. Describe...
-
A retail store controls its inventoried items using the reorder point method; however, the shelf space for these items is limited. (Retailers allocate a certain amount of shelf space to each item in...
-
A glass manufacturer produces hand mirrors. Each mirror is supposed to meet company standards for such things as glass thickness, ability to reflect, size of handle, quality of glass, color of...
-
A person is riding in a car traveling on a straight level road. What is the direction of the net force on the person if the car is (a) Speeding up, (b) Slowing down, (c) Has a constant speed?
-
Give examples of objects whose motion is described by the plots in Figure P2.20. Figure P2.20 Case 2 Case 3 Case 1
-
Two football players start running at opposite ends of a football field (opposite goal lines), run toward each other, and then collide at the center of the field. They start from rest and are running...
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


