Some liquid water is placed in a room-temperature container and sealed. After some time, the level of
Question:
Some liquid water is placed in a room-temperature container and sealed. After some time, the level of the liquid drops by a small amount. After that, the water level remains constant.
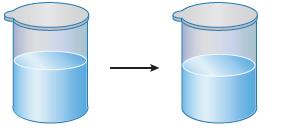
What does this have to do with equilibrium?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
This scenario relates directly to the concept of equilibrium Heres the breakdown Initial State Water ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
A container filled with 45 kg of liquid water at 95°C is placed in a 90-m3 room that is initially at 12°C. Thermal equilibrium is established after a while as a result of heat transfer...
-
A container filled with 45 kg of liquid water at 95°C is placed in a 90-m3 room that is initially at 12°C. Thermal equilibrium is established after a while as a result of heat transfer...
-
Some water is placed in a sealed glass container connected to a vacuum pump (a device used to pump gases from a container), and the pump is turned on. The water appears to boil and then freezes....
-
Using atomic weight, crystal structure, and atomic radius data tabulated inside the front cover of the book, compute the theoretical densities of aluminum (Al), nickel (Ni), magnesium (Mg), and...
-
Analyzing and Interpreting Return on Assets Tiffany & Co. is one of the worlds premier jewelers and a designer of other fine gifts and housewares. Presented here are selected income statement and...
-
What are the advantages of using virtual queues?
-
Jamie Lee has decided to purchase a certified, preowned vehicle. What might she expect as far as reliability and a warranty on the used car?
-
Click a Pix Photography works weddings and prom-type parties. The balance of retained earnings was $26,000 at December 31, 2015. At December 31, 2016, the business's accounting records show these...
-
Old MathJax webview can you please help me to do a comparative balance sheet, income statement and calculation of materiality . Apollo Shoes, Inc Trial Balance (Audited) 31-Dec-19 PBC A-T Credit 0 0...
-
Consider the reversible reaction shown below: Instead of using molar concentrations, you can use gas pressures to calculate an equilibrium constant, in which case it is often labeled K p instead of K...
-
A sealed 1-L flask is filled with 1 mole of H 2 (g) and 1 mole of I 2 (g). The reversible reaction H 2 (g) + I 2 (g) 2HI(g) ensues. A plot showing what happens to the HI concentration as a percent...
-
Provide examples of each of the following: (a) direct labour, (b) indirect labour, (c) direct materials, (d) indirect materials and (e) indirect expenses. (op. 27-28)
-
Consider how they might directly apply to your life and work environment when answering the questions below. Competency 1: Evaluate data-driven processes and approaches of an organization's...
-
There are several website optimizer tools available to help you "increase website conversion rates." Following : Explain fully what is meant by "increase website conversion rates"; then, identify two...
-
Imagine being a human resource director for a large hotel chain. Report to management highlighting problematic diversity issues that may arise. Identify 3 challenging diversity issues (e.g., race,...
-
A study based on a sample of 4 0 0 medical school students finds that the ratio of female students is 0 . 4 8 . The school rules require that the female ratio in the school is at least 0 . 5 ? a ) (...
-
One of the many paradoxes in leadership is the challenge of encouraging a team effort while simultaneously encouraging individuals to excel. Why is this paradox a challenge for team leaders, and how...
-
A large hospital uses a certain intravenous solution that it maintains in inventory. Pertinent data about this item are as follows: a. Design a reorder point system of control for this item. b....
-
Why is it necessary to study the diffusion of molecules in biological systems?
-
In SI units, acceleration is measured in units of meters per second squared (m/s 2 ). Which of the following combinations of units can also be used to measure acceleration? (a) Cm/s (b) Cm/s 2 (c) m...
-
Consider the motion of a sprinter running a 100-m dash. When it is run outdoors, this race is run along a straight-line portion of a track, so it is an example of motion in one dimension. Draw...
-
A car is initially at rest on an icy road that is extremely slippery. The driver finds that he is unable to get the car to drive away because the wheels simply spin when he tries to accelerate....
-
Question 4. - Week 9. What are the major competitive issues General Electric faces when managing cooperative strategies? - (7 marks)
-
All of the following are roles of a derivative exchange EXCEPT: _____. A) maintaining margin requirements on futures contracts B) reducing the default risk on forward contracts C) performing daily...
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...

Study smarter with the SolutionInn App


