The boiling point of one of these compounds is 24 C, and the boiling point of the
Question:
The boiling point of one of these compounds is –24 °C, and the boiling point of the other is 78°C:
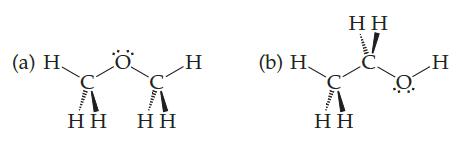
Which boils at which temperature? Why? If hydrogen bonds are involved in either case, make a drawing using dotted lines to show the hydrogen bonding
Transcribed Image Text:
(a) H Η Η Ö Η Η H (b) Η, Η Η Η Η Ο H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Compound b boils at 78 C and compound a boils at24 C The reason cant be London forces beca...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
The temperature at which water boils (the boiling point) depends on elevation: The higher the elevation, the lower the boiling point will be. At sea level, water boils at 212F; at an elevation of...
-
At sea level, water boils at a temperature of 212F. As the altitude increases, the boiling point of water decreases. For instance, at an altitude of 5000 feet, water boils at about 202.8F. (a) Find a...
-
Glorious Electrical Appliances (GEP) Co. is a company that sells electrical tools. GEP uses perpetual inventory system in recording its inventory. The financial position of GEP as at 31 December 2016...
-
H. S. Black is noted for developing a negative feedback amplifier in 1927. Often overlooked is the fact that three years earlier he had invented a circuit design technique known as feed forward...
-
Mass communications will play a key role in Sonics product introduction.After reviewing your earlier decisions and thinking about the current situation (especially your competitive circumstances),...
-
What organizational changes are necessary in order to implement the long-term plan derived in question number three above?
-
Consider the trends as analyzed in the last section. Why, in your opinion, are greenfield investments are on the rise? What other changes can be expected in the coming years in terms of the Chinese...
-
Alitech Corp. is a small midwestern business that owns a valuable patent. Alitech has approximately 1,000 shareholders with 100,000 authorized and outstanding shares. Block Corp. would like to have...
-
The two major bond rating agencies are Moody's and S&P l. Explain how they rate bonds and why it's important?
-
Draw a picture showing how NH 3 molecules attract one another.
-
The molecules in a gas phase will (a) Speed up upon heating the gas. (b) Slow down upon heating the gas. (c) Change phase upon sufficient heating of the gas. (d) Form a condensed phase upon...
-
Suppose management has left you out of the requirements definition process for the development of the system in question
-
The break even point of a company is $240 000. They sell their product at a markup of 30% and have variable expenses of 9% of sales. They currently make a profit of $10 500. They plan on reducing...
-
What kind of messages are young girls and boys receiving about whether their safety in relationships is valued are by their families and communities? Do we emphasize to our children (boys and girls)...
-
Below are the transactions for Oliver Printing, Incorporated for June, the first month of operations. June 1 Obtain a loan of $ 5 6 , 0 0 0 from the bank by signing a note. June 2 Issue common stock...
-
Assume an organization must invest $ 7 0 0 , 0 0 0 in fixed costs to produce a product that sells for $ 7 5 and requires $ 4 0 in variable costs to produce one unit. What is the organization s...
-
hotel delta marriott montreal What do you think is the value and purpose for the hotel brand choosing to make CSR an important part of their overall business strategy? What two recommendations based...
-
Find the equation of the parabola with vertex at the origin and axis along the x-axis if the parabola passes through the point (3, -1). Make a sketch.
-
What can you do to reduce hunger where you live? To reduce hunger globally?
-
Identify the reagents necessary to make each of the following amino acids using a HellVolhardZelinski reaction. (a) Leucine (b) Alanine (c) Valine
-
Draw the aldehyde that is obtained as a byproduct when l-leucine is treated with ninhydrin.
-
Identify the starting alkene necessary to make each of the following amino acids using an asymmetric catalytic hydrogenation. (a) l-alanine (b) l-valine (c) l-leucine (d) l-tyrosine
-
Summarize in your own words Sharps, Treynors, and Jensens Measures for assessing portfolio performance with respect to risk. Assess the portfolio performance of mutual fund VDIGX taking into...
-
Question 1 Slat and Company have recently set up a business which will manufacture and sell a furniture component, the F12 On the 19 August 2021, the company issued 85,000 of share capital for cash....
-
The following is Addison Corporations contribution format income statements for last month. The company has no beginning or ending inventories. A total of 10,000 units were produced and sold last...

Study smarter with the SolutionInn App


