The compound CH 3 COOCH 3 (methyl acetate) is not an acid, but the compound CH 3
Question:
The compound CH3COOCH3 (methyl acetate) is not an acid, but the compound CH3COOH (acetic acid) is.
(a) Which of the hydrogen atoms in acetic acid is the acidic hydrogen (the one that becomes H+ (aq) ion)?
(b) Speculate about why none of the hydrogen atoms in methyl acetate is acidic.
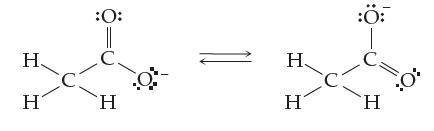
(c) The diagram below shows how electrons rearrange to make acetic acid behave as an acid. Notice the two different ways the resulting anion is drawn. This allows the negative charge to be spread among two oxygen atoms, which in turn makes the whole anion stable and helps acetic acid to lose an H+ ion and be an acid. What do you call the two different forms of the anion?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





