Write the complete ionic equation and the net ionic equation for the reaction that occurs when the
Question:
Write the complete ionic equation and the net ionic equation for the reaction that occurs when the following solutions are mixed, and name the salt formed in each case:
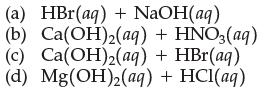
Transcribed Image Text:
(a) HBr(aq) + NaOH(aq) (b) Ca(OH)2(aq) + HNO3(aq) (c) Ca(OH)₂(aq) + HBr(aq) (d) Mg(OH)2(aq) + HCl(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a HBraq NaOHaq Complete ionic equation Haq Braq Naaq OHaq Naaq Braq HOl Net ionic eq...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Write the complete ionic equation and the net ionic equation for the reaction that occurs between aqueous solutions of: (a) Silver nitrate and potassium iodide (b) Lithium sulfate and silver acetate
-
Magnesium carbonate, magnesium oxide, and magnesium hydroxide are all white solids that react with acidic solutions. (a) Write a balanced molecular equation and a net ionic equation for the reaction...
-
Write the complete ionic equation for the reaction of FeCl2(aq) and AgNO3(aq). You may have to consult the solubility rules.
-
How would your answers in Problem 48 change if partnership revenues were $100,000 instead of $150,000? Data From Problem 48: The KL Partnership is owned equally by Kayla and Lisa. Kaylas basis is...
-
Jensen Company makes a product that sells for $38 per unit. The company pays $16 per unit for the variable costs of the product and incurs annual fixed costs of $176,000. Jensen expects to sell...
-
Define the term key performance indicator. The text provides you with examples of KPIs applicable to revenue cycle activities. Extend that line of thinking and identify at least five possible KPIs...
-
Can you think of any circumstances where deferring conflict might be a wise course of action? LO6
-
At the end of June, Morton Company had a balance of $49,900 in the vacation benefits payable account. During July, employees earned an additional $3,110 in vacation benefits, but some employees used...
-
Break-Even Units, Contribution Margin Ratio, Margin of Safety Khumbu Company's projected profit for the coming year is as follows: Total Per Unit Sales $1,086,500 $26.50 Total variable cost 499,790...
-
Consider a gas-phase reaction. (a) Cooling the mixture of reactants can slow and even stop the chemical reaction. Explain why this is so. (b) Increasing the number of reactant molecules in the flask...
-
The compound CH 3 COOCH 3 (methyl acetate) is not an acid, but the compound CH 3 COOH (acetic acid) is. (a) Which of the hydrogen atoms in acetic acid is the acidic hydrogen (the one that becomes H +...
-
(a) Show that the skin depth in a poor conductor ( < < w) is (2/)/ (independent of frequency). Find the skin depth (in meters) for (pure) water. (b) Show that the skin depth in a good conductor ( >>...
-
Popcorn company is expected to pay $1 dividend per share at the end of this year, $1.50 dividend per share at the end of year 2, $2 dividend per share at the end of year 3, and $2.50 dividend per...
-
Increased spending for COVID economic relief is an important issue for many struggling in the current economy. A specific policy to combat this issue is put forward and it is found that 78% of...
-
James worked a total of 186 hours for the month of June 2020. His rate per hour is working hours of the 450 per hour. Overtime premium is 30%. The company is 8 hours a day. The company's regular...
-
Question Researchers collected a simple random sample of 36 children who had been identified as gifted in a large city. The following histograms show the distributions of the IQ scores of mothers and...
-
Shown below is activity for one of the products of Denver Office Equipment: January 1 balance, 700 units @ $55 per unit $38,500 Purchases: January 10: 700 units @ $60 per unit January 20: 1,100 units...
-
a. At one of the following ions is purple and the other is blue. Which is which? b. What would be the difference in the colors of the compounds at pH = 3? CH3)2N N(CH3)2
-
Briefly discuss the implications of the financial statement presentation project for the reporting of stockholders equity.
-
Figure P.9.43 illustrates a setup used for testing lenses. Show that when d 1 and d 2 are negligible in comparison with 2R 1 and 2R 2 , respectively. (Recall the theorem from plane geometry that...
-
A wedge-shaped air film between two flat sheets of glass is illuminated from above by sodium light ( 0 = 589.3 nm). How thick will the film be at the center of the 173rd bright fringe (counted from...
-
Suppose a wedge-shaped air film is made between two sheets of glass, with a piece of paper 7.618 10 -5 m thick used as the spacer at their very ends. If light of wavelength 500 nm comes down from...
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...
-
Selk Steel Co., which began operations on January 4, 2017, had the following subsequent transactions and events in its long-term investments. 2017 Jan. 5 Selk purchased 50,000 shares (25% of total)...
-
Equipment with a book value of $84,000 and an original cost of $166,000 was sold at a loss of $36,000. Paid $100,000 cash for a new truck. Sold land costing $330,000 for $415,000 cash, yielding a...

Study smarter with the SolutionInn App


