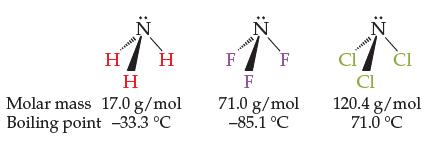
The three molecules below increase steadily in molar mass from left to right. However, their boiling points
Question:
The three molecules below increase steadily in molar mass from left to right. However, their boiling points do not. Explain why the boiling points do not also steadily increase from left to right.
Transcribed Image Text:
H H H Molar mass 17.0 g/mol Boiling point -33.3 °C F :2 F F 71.0 g/mol -85.1 °C CI CI CI 120.4 g/mol 71.0 °C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
The trend in boiling points of the molecules N2 HF and Cl2 doesnt strictly follow the increase in mo...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Write a literature review for your study. See below for an example of a literature review. Your literature review should provide both analysis and synthesis of previous studies as related to the...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Holly needs $21,800 worth of new equipment for his shop. He can borrow this money at a discount rate of 11% for a year. Find the amount of the loan Holly should ask for so that the proceeds are...
-
Discuss why making changes such as Mitsubishi did is important both legally and for improving HR management with the employees and managers.
-
Rose dies with passive activity property having an adjusted basis of $65,000, suspended losses of $13,000, and a fair market value at the date of her death of $90,000. Of the $13,000 suspended loss...
-
What is a CF?
-
Redraft the following issues in the format presented in this chapter. Part A Can a bystander who witnesses the death of a victim from three blocks away recover for negligent infliction of emotional...
-
If you think a company's stock will fall moderately from it's current price of $100, and you Buy ATM Put Strike: $100 Premium: $7 and you Sell an OTM Put strike:$75, Premium: $3 The net premium you...
-
What is wrong with the following diagram when it comes to explaining London forces?
-
Arrange in order of increasing boiling point: CO 2 , SO 2 , CH 3 CH 2 OH, Al.
-
Describe the global character of the ocean and its importance to life on Earth in terms of the effects of mismanagement of the bluefin tuna fishery in the Mediterranean or the expansion of the Gulf...
-
You are an external auditor in a firm that undertakes the audit of Canadian Life and Mutual (CLM), a large, Montreal-based financial institution. CLM relies heavily on its computer-based information...
-
You need to temporarily increase the feed rate to an existing column without flooding. Since the column is now operating at about \(90 \%\) of flooding, you must vary some operating parameter. The...
-
Consider, again, the clothing data set. Obtain the three summary plots of the sample cross-correlations for lags 1 to 21.
-
Based on the dangling-else discussion in Exercise 3.27, modify the following code to produce the output shown. Use proper indentation techniques. You must not make any additional changes other than...
-
Consider the random process \(U(t)=A\), where \(A\) is a random variable uniformly distributed on \((-1,1)\). (a) Sketch some sample functions of this process. (b) Find the time autocorrelation...
-
Find the area of the surface generated by revolving the curve x = 2 + cos t, y = 1 + sin t, for 0 t 2 about the x-axis.
-
Why is it important to understand the macro-environment when making decisions about an international retail venture?
-
You are a NASA engineer faced with the task of ensuring that the material on the hull of a spacecraft can withstand puncturing by space debris. The initial cabin air pressure in the craft of 1 atm...
-
Describe the random-walk model of diffusion. How is this model related to Brownian motion?
-
In the StokesEinstein equation that describes particle diffusion for a spherical particle, how does the diffusion coefficient depend on fluid viscosity and particle size?
-
A firm purchased a new piece of equipment with an estimated useful life of eight years. The cost of the equipment was $65,000. The salvage value was estimated to be $10,000 at the end of year 8....
-
5. Which of the following is the cheapest for a borrower? a. 6.7% annual money market basis b. 6.7% semi-annual money market basis c. 6.7% annual bond basis d. 6.7% semi-annual bond basis.
-
Waterloo Industries pays 30 percent corporate income taxes, and its after-tax MARR is 24 percent. A project has a before-tax IRR of 26 percent. Should the project be approved? What would your...

Study smarter with the SolutionInn App


