These three substances are all liquids at room temperature: Which do you expect to be least soluble
Question:
These three substances are all liquids at room temperature:
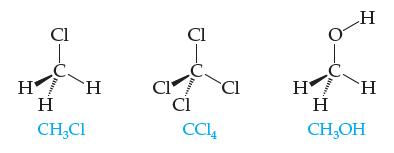
Which do you expect to be least soluble in water? Most soluble in water? Explain your answers fully
Transcribed Image Text:
Cl HEH Η H CH₂Cl CI Cl B Cl CC14 Cl H 1000 H H H CH₂OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
expect methanol CH3OH to be the most soluble in water and carbon tetrachloride CCl4 to be the least soluble in water Explanation Water is a polar mole...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
You are analyzing various programs to reduce water pollution from food processing plants. In consultation with your staff, you have developed the following matrix of effects (where PV = Present...
-
Which do you expect to be more sustainable: grazing on public land or grazing on privately owned pastures? Why?
-
Capital Inc. has prepared the operating budget for the first quarter of 2015. They forecast sales of $50,000 in January, $60,000 in February, and $70,000 in March. Variable and fixed expenses are as...
-
JOURNAL ENTRIES FOR MATERIAL, LABOR, AND OVERHEAD Jones Manufacturing Corporation had the following transactions for its job order costing operation. Prepare general journal entries to record these...
-
Why is being a member of a cross-functional team considered to be helpful experience as a future leader?
-
5. Scholarships are classified as: a Academic support b Student services c Institutional support d Student aid
-
Define experimental condition, experimental treatment, and experimental group. How are these related to the implementation of a valid manipulation?
-
QBO: Quick Books Online QUESTION 1 Which of the following statement is not true? O a Budgets by class or location can be created in QBO O b. Users can set up a budget from scratch or can have QBO...
-
When a liquid solute dissolves in water, there is still a solute-separation step that absorbs energy, but the step doesnt require breaking up a crystal lattice as for a typical solid solute. What...
-
When a gaseous solute dissolves in water, which step in the dissolving process is essentially skipped? Explain why.
-
Read Case 1.1, which focuses on RunKeeper. What similarities, if any, do you see between RunKeepers start-up story and the start-up stories of Rovio Mobile (the company behind Angry Birds) and Zeo,...
-
Most businesses have been impacted negatively in 2020 by the outbreak of Corona virus leading to the disease Covid 19. Many countries went in lock down where by economic activities nearly came to a...
-
The unadjusted trial balance has been entered on a 10-column end-of-period spreadsheet work sheet) for you. Complete the spreadsheet using the following adjustment data a Physcial inventory count on...
-
A) What should be the price of the call option? B) Assume that the call option on Apple with strike price $90 and maturity in one year is currently trading at $17. You immediately tell your broker...
-
White Company has two departments, Cutting and Finishing. The company uses job-order costing and computes a predetermined overhead rate in each department. The Cutting Department bases its rate on...
-
Can someone please help me figure out how to find the qualified business income for this problem? Maria and Javier are the equal partners in MarJa, a partnership that is a qualifying trade or...
-
Evaluate by cursory inspection: det ab 2b c+b
-
Do public and private companies follow the same set of accounting rules? Explain.
-
The steel shaft has a radius of 15 mm. Determine the torque T in the shaft if the two strain gages, attached to the surface of the shaft, report strains of ε x² = -80(10 -6 ) and...
-
The shaft has a radius of 15 mm and is made of L2 tool steel. Determine the strains in the x² and y² direction if a torque T = 2 kN · m is applied to the shaft. 45 VT
-
The A-36 steel pipe is subjected to the axial loading of 60 kN. Determine the change in volume of the material after the load is applied. 30 mm 40 mm 60 kIN 60 kN 0.5 m
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


