Two reaction-energy profiles are shown below. (a) At a given temperature, which reaction is faster, the one
Question:
Two reaction-energy profiles are shown below.
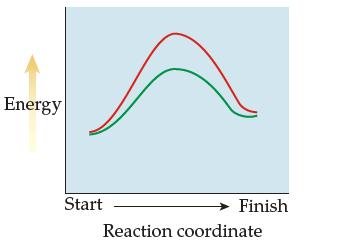
(a) At a given temperature, which reaction is faster, the one depicted in red or the one depicted in green? Explain.
(b) Which reaction is exothermic?
Transcribed Image Text:
Energy Start Finish Reaction coordinate
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
The image you sent shows two reactionenergy profiles The red profile ...View the full answer

Answered By

Ashok Kumar Malhotra
Chartered Accountant - Accounting and Management Accounting for 15 years.
QuickBooks Online - Certified ProAdvisor (Advance - QuickBooks Online for 3 years.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
During 2 0 2 4 , its first year of operations, Riley Construction provides services on account of $ 1 2 6 , 0 0 0 . By the end of 2 0 2 4 , cash collections on these accounts total $ 9 3 , 0 0 0 ....
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Which property of the group 6A elements might be the one depicted in the graph shown here: (a) Electronegativity (b) First ionization energy (c) Density (d) X-X single-bond enthalpy (e) Electron...
-
Consider the following chair conformation of bromocyclohexane: (a) Identify whether the bromine atom occupies an axial position or an equatorial position in the conformation above. (b) Draw a...
-
Analyzing the Effects of Transactions Using T-Accounts, Preparing a Balance Sheet, and Evaluating the Current Ratio over Time as a Bank Loan Officer Strauderman Delivery Company, Inc., was organized...
-
Reconsider the savingsincome regression in Section 8.7. Suppose we divide the sample into two periods as 19701982 and 19831995. Using the Chow test, decide if there is a structural change in the...
-
Post, Inc., had a receivable from a foreign customer that is payable in the customers local currency. On December 31, 2009, Post correctly included this receivable for 200,000 local currency units...
-
Instantaneous Power in a Standing Wave. From Eq. (15.21), the instantaneous rate at which a wave transmits energy along a string (instantaneous power) is Where F is the tension. (a) Evaluate f (x, t}...
-
Grohl Co. issued 5-year bonds a year ago at a coupon rate of 9 percent. The bonds make semiannual payments. If the YTM on these bonds is 10 percent, what is the current bond price? Multiple Choice...
-
In which vessel is the gas phase reaction A + B P being run at a higher concentration, and in which vessel is the reaction rate greatest? Explain why. (Both vessels are at the same temperature.) A +...
-
Consider a gas-phase chemical reaction. The three situations depicted represent the reaction being run at different temperatures. (a) Which of the depictions represents the reaction being run at the...
-
Explain what care business people must exercise when entering into contracts with government corporations or bodies.
-
5) A frictionless rod of length L rotates counterclockwise in the with constant angular speed w at an angle a to the z axis. A bead of mass m, free to slide on the rod, leaves the origin with initial...
-
1) Louisa is a corn farmer in Illinois. She anticipates a harvest in August of 3 million bushels of yellow corn. Today is May. Louise plans to hedge her sale of corn in August using corn futures...
-
2. DETAILS MY NOTES In a statistical test, we have a choice of a left-tailed test, a right-tailed test, or a two-tailed test. Is it the null hypothesis or the alternate hypothesis that determines...
-
2. The model of a two-story building shown in Figure 2. The girders are assumed to be rigid, and the columns have flexural rigidities EI and EI2, with negligible masses. The stiffness of each column...
-
Prepare journal entries to record these transactions. (List all debit entries before credit entries. Credit account titles are automatically indented when amount is entered. Do not indent manually....
-
Let B be any matrix that satisfies Penrose conditions 1 to 3 and let x = Bb. Show that x is a solution to the normal equations ATAx = ATb.
-
Reduction in sales All of the above 29. Belt of an electric motor is broken, it needs a. Corrective maintenance b. Scheduled maintenance c. Preventive maintenance d. Timely maintenance. 30. The...
-
A hot air balloon is needed to lift a load with a mass of 125 kg from the earths surface. If the ambient air is 20C and the air in the balloon can be heated to 110C, determine the required diameter...
-
A scuba diver with wet suit, tank, and gear has a mass of 78 kg. The diver and gear displace a total volume of 82.5 L of sea water. The diver would like to add enough lead weights to become neutrally...
-
Concrete is to be poured for a large foundation, but a round passageway is required through the concrete to carry utilities through it. A lightweight plastic tube will be placed horizontally in the...
-
Chapter o Homew ebook 50,000-unit production quantity: $ 227,049 7 70,000-unit production quantity: $ 66,751 d. In addition to mean profit, what other factors should FTC consider in determining a...
-
Diamond makes downhill ski equipment. Assume that comic has offered to produce ski poles for Diamond for $20 per pair Diamond needs 200,000 pairs of poles per period Diamond can only avoid 5150,000...
-
17? Which of the following statement is true Select one: a. All evidence must have the same level of reliability b. All evidence must have the same level of persuasiveness C. All are false d....

Study smarter with the SolutionInn App


