Consider a gas-phase chemical reaction. The three situations depicted represent the reaction being run at different temperatures.
Question:
Consider a gas-phase chemical reaction. The three situations depicted represent the reaction being run at different temperatures.
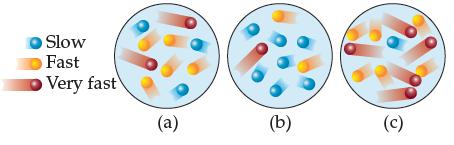
(a) Which of the depictions represents the reaction being run at the lowest temperature?
(b) Which of the depictions would lead to the reaction proceeding at the slowest rate, and why?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





