What is the volume in milliliters of 15.0 g of (a) Liquid water at 25C, (b) Ice
Question:
What is the volume in milliliters of 15.0 g of
(a) Liquid water at 25°C,
(b) Ice at 0°C,
(c) Gasoline at 25°C,
(d) Lead at 25°C,
(e) Mercury at 25°C,
(f) Helium gas at 0°C and 1 atm pressure?
(Use data from Table 2.4.)
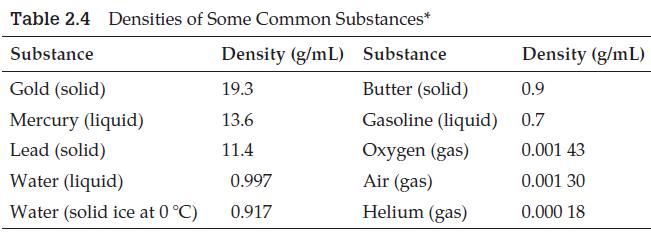
Transcribed Image Text:
Table 2.4 Densities of Some Common Substances* Substance Density (g/mL) Substance Gold (solid) 19.3 Butter (solid) Mercury (liquid) 13.6 Gasoline (liquid) Lead (solid) 11.4 Oxygen (gas) Water (liquid) Air (gas) Water (solid ice at 0 °C) Helium (gas) 0.997 0.917 Density (g/mL) 0.9 0.7 0.001 43 0.001 30 0.000 18
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a The density of liquid water at 25C is 0997 gmL so the volume of 150 g of liquid water a...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Determine x (1) 2 for the system of equations using GaussSeidel with an initial guess of x 1 = 0, x 2 = 0, x 3 = 0. 3 24 X1 [CHO 1 30 X2 24 1 X3 6 3 4
-
Liquid water at 25 C and 1 bar fills a rigid vessel. If heat is added to the water until its temperature reaches 50 C, what pressure is developed? The average value of ( between 25 and 50oC is 36.2 (...
-
A firm has total debt of $6,000,000 and stockholder's equity is $4,000,000. The firm wants to calculate equity-to- total asset ratio in order to make decision about further raise of capital. What is...
-
What are the advantages and disadvantages of team-written documents?
-
Suppose that Bob decides to continue to make $1,000 deposits into his IRA every year until his 65th birthday. If John still waits until he is 36 to start his IRA, how much must he deposit each year...
-
What is gross operating income?
-
Refer to the Johnson Filtration problem introduced in this section. Suppose that in addition to information on the number of months since the machine was serviced and whether a mechanical or an...
-
The nail salon in the strip mall has begun working with their owner's son, who is a marketer. He suggested the nail salon walk through the marketing research process, and they are now into the...
-
The mass of an average neon atom is 20.2 atomic mass units (amu), where 1 amu = 1.66 10 24 g. (a) What is the mass in atomic mass units of 20 neon atoms? (b) What is the mass in grams of 20 neon...
-
A block measures 6.0 cm on each side. What is the volume of the block in cubic meters?
-
In the audit of the BCP, we recommend that someone who was not associated with the writing of the plan do the actual review. Then, again, in some of the exercises to test the BCP, we recommend that...
-
The residents of the town of Stewart are well-known coffee drinkers. Dunkin Donuts and Starbucks stores supply the residents with over 100,000 cups of hot coffee per week. Recently, coffee drinkers...
-
Read through the following case study and analyse it by addressing the question that follows. Case study: Adriana Adriana runs a very busy multi-sports coaching company that provides after-school...
-
Assuming that you were invited to one of your best friends' wedding. Therefore, you decided to purchase a special product or service as a gift for this important event. Based on this scenario, you...
-
The Department of Commerce, through the Economic Development Administration (EDA), is seeking information to inform the planning and design of the Regional Technology and Innovation Hub (Tech Hubs)...
-
In frontline management, it is essential to manage time effectively. In the table below, list 3 time-management principles that are relevant to a frontline manager's own work and provide 3 examples...
-
Describe the location and structure of the juxtaglomerular apparatus.
-
Discuss the information available from the following techniques in the analysis of inorganic pigments used in antique oil paintings: (i) Powder X-ray diffraction, (ii) Infrared and Raman...
-
Which of the following compounds below do you expect to have a longer λ max ?
-
Predict the expected λ max of the following compound:
-
When 5-deutero-5-methyl-1,3-cyclopentadiene is warmed to room temperature, it rapidly rearranges, giving an equilibrium mixture containing the original compound as well as two others. Propose a...
-
Phantom Consulting Inc. is a small computer consulting business. The company is organized as a corporation and provides consulting services, computer system installations, and custom program...
-
Sam owns a 25% in Spade, LLC. In 2021, Spade reports $100,000 or ordinary income. What is Sams qualified business income (QBI) deduction? answer is 5,000 but please show how to get it
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...

Study smarter with the SolutionInn App


