Which of the following are electron-transfer reactions? (a) 2 CrO2 + 2 HCrO72 + HO (b) Fe
Question:
Which of the following are electron-transfer reactions?
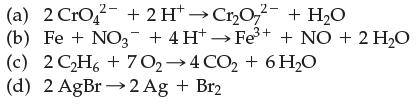
Transcribed Image Text:
(a) 2 CrO2 + 2 H→Cr₂O72 + H₂O (b) Fe + NO3+ 4H+→Fe³+ + NO + 2 H₂O (c) (d) 2AgBr→2Ag + Br2 2C2H6+7O,→4CO,+6H,O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
Of the following reactions in the image a 2 CrO22H Cr2O7 HO b Fe NO3 4H Fe3 NO 2 H2O c 2CH7O 4CO 6HO ...View the full answer

Answered By

Rajat Gupta
used to take tution classes from my school time.
Conducted special topic claases during my graduation to help the students pass their exams.
Currently, teaching and conducting online claases during my post- graduation too.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Which of the following proposed reactions would take place quickly under mild conditions? (a) (b) (c) (d) (e) (CHJ2CH-C-NH, + CH3OH (CHJ-CH-C-OCH, + NH, CH,CH,--C-Cl + CH3-C-OH CH,CH-_C_O_C_CH, + HCI
-
The following are electron configurations for some ions. Which ones would you expect to see in chemical compounds? State the concept or rule you used to decide for or against any ion. a. Fe2+...
-
Which of the following reactions occurs most rapidly? Why? a. b. c. Br - H20 C(CH3)3 C(CH3)a (CH)a C(CH3)3 Br - H20 CH33 C(CH3)3
-
Marc & Kent operate a vineyard as partners. The partnership trades under the business name Victor Estate. Victor Estate has prepared a general ledger for the 2023 income year showing the following...
-
What is the first step in preparing a master budget?
-
What effective leadership behaviors did Colonel Novak exhibit?
-
Ethical Dilemma: Censorship standards vary worldwide. What is acceptable in some countries, such as nudity on television, criticizing authority, or revealing government secrets that affect national...
-
Early in 2011, the Excalibur Company began developing a new software package to be marketed. The project was completed in December 2011 at a cost of $6 million. Of this amount, $4 million was spent...
-
Please answer the following question Business transactions completed by Hannah Venedict during the month of September are as follows. a . Venedict invested $ 6 0 , 0 0 0 cash along with office...
-
Which of the following are redox reactions? (a) 2Na + 2H 2 O 2NaOH + H 2 (b) MgBr 2 + 2NaF MgF 2 + 2NaBr (c) 2CO + O 2 2CO 2 (d) SO 2 + H 2 O H 2 SO 3
-
Why can we always call an electron-transfer reaction a redox reaction?
-
Know the difference between tangible and intangible resources.
-
In 2024, the Westgate Construction Company entered into a contract to construct a road for Santa Clara County for $10,000,000. The road was completed in 2026. Information related to the contract is...
-
Briefly describe the case you have chosen. Categorize the social worker's experience as vicarious trauma, compassion fatigue, or burnout. Provide justification. Identify the social worker's score on...
-
Given f(x) below, find f'(x). f(x) = = m 5z In (2) et dt
-
Olsen & Alain, CPAs (O&A) performed the audit of Rocky Point Brewery (RPB), a public company in 20X1 and 20X2. In 20X2, O&A also performed tax services for the company. Which statement best describes...
-
Exercise 9-4 (Algo) Prepare a Flexible Budget Performance Report [LO9-4] Vulcan Flyovers offers scenic overflights of Mount Saint Helens, the volcano in Washington State that explosively erupted in...
-
Let A be a 4 Ã 4 matrix with reduced row echelon form given by If determine a3 and a4. 2100 1000 4371 3521 al
-
What can scientists learn by comparing the fossilized skeletons of extinct primates with the bones of modern species?
-
A meniscus concave glass (n l = 1.5) thin lens (see Fig. 5.12) has radii of curvature of +20.0 cm and +10.0 cm. If an object is placed 20.0 cm in front of the lens, show that the image distance will...
-
Going back to Section 5.2.3, prove that for a thin lens immersed in a medium of index n m That done, imagine a double-concave air lens surrounded by water; determine if its converging or diverging. (...
-
A biconvex glass (n 1 = 1.5) thin lens is to have a +10.0-cm focal length. If the radius of curvature of each surface is measured to be the same, what must it be? Show that a spider standing 1.0 cm...
-
On August 1 , 2 0 2 3 , Mark Diamond began a tour company in the Northwest Territories called Millennium Arctic Tours. The following occurred during the first month of operations: Aug. 1 Purchased...
-
A company manufactures lawnmowers. Compute the total amount of period costs from thr following costs.
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...

Study smarter with the SolutionInn App


