Which of the following represent a chemical transformation? (a) 4 P(s) + 5O(g) 2 PO5(s) (b)
Question:
Which of the following represent a chemical transformation?
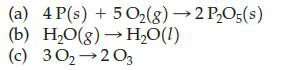
Transcribed Image Text:
(a) 4 P(s) + 5O₂(g) → 2 P₂O5(s) (b) H₂O(g) → H₂O(1) (c) 30₂ 203
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a and c because the produc...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Determine which of the following represent potential energy and which represent kinetic energy. (a). Thermal energy (b). Gravitational energy (c). Chemical energy (d). Electrostatic energy
-
Which of the following represent configurations of thallium ions in compounds? Explain your decision in each case. a. Tl2+ [Xe]4f145d106p1 b. Tl3+ [Xe]4f145d10 c. Tl4+ [Xe]4f145d9 d. Tl+...
-
Which of the following structures represent the same compound? Which ones represent different compounds? (a) (b) (c) (d) (e) (f) (g) Name the structures given in Problem 3-33, parts (a), (c), (e),...
-
Sugar (C12H22O11) is a molecular compound that stays together inwater, while NaCl and MgSO4?7H2O are ionic compounds that dissociate into cations andanions as illustrated in the NaCl example below:...
-
Locate the 2007 financial statements for The Walt Disney Company on the Internet. Use those financial statements and consider the following questions. 1. As illustrated in Exhibit 10-10, Interbrand...
-
What are the issues of using one full-service agency versus multiple specialized agencies?
-
Assume X is an It process. Use Its formula to derive the following: (a) Define Yt = eXt . Show that dY Y = dX + 1 2 (dX) 2 . (b) Assume X is strictly positive. Define Yt = logXt. Show that dY = dX X ...
-
Mike Samson is a college football coach making a base salary of $652,800 a year ($54,400 per month). Employers are required to withhold a 6.2% Social Security tax up to a maximum base amount and a...
-
Case: Transaction Analysis for Nike, Inc. We all know abou Nike! The largest seller of athletic footwear and apparel in the world! Please refer to the 2020 Nike, Inc. 10-K, the income statement (they...
-
Water (H 2 O) and carbon dioxide (CO 2 ) are produced in a chemical reaction when methane (CH 4 ) and oxygen (O 2 ) are combined and heated. Which are the reactants, and which are the products?
-
You are presented with a block made of some pure metal and told the metal is gold, but you have your doubts. Using a thermometer, how can you determine whether the metal is gold?
-
Overland Steel operates a coal- burning steel mill in New York State. Changes in the states air qual-ity control laws will result in this mills incurring a $ 1,000 per day fine ( which will be paid...
-
Because her insurance agency is in a lakeside community, Adriana always asks her homeowners clients what boating activities they engage in, and she is sure to add the Watercraft endorsement...
-
1. Among all assumptions in CVP analysis, which one do you think is the most critical? Explain. 2. How will you change the cost-volume-profit analysis if the assumption (you identify in the previous...
-
Construct a confidence interval for p-P2 at the given level of confidence. x =26, n =229, x2 = 31, n = 302, 95% confidence The researchers are % confident the difference between the two population...
-
AP 9-2 (Moving Expenses) In May of the current year, following a dispute with her immediate superior, Ms. Elaine Fox resigned from her job in Halifax and began to look for other employment. She was...
-
Minimize the number of states in the following DFA: A b b a a a b b b E B a a
-
Explain the splitting patterns in 1HNMR spectrum D in detail. The insets are fivefold expansions
-
For each of the following transactions, indicate whether it increases, decreases, or has no effect on the following financial ratios: current ratio, debt-to-equity ratio, profit margin ratio, and...
-
How many constitutional isomers are obtained when each of the following compounds undergoes monochlorination? (a) (b) (c) (d) (e) (f) (g) (h) (i) (j)
-
Propylene is produced by cracking petroleum and is a very useful precursor in the production of many useful polymers. Propylene has one constitutional isomer. Draw that isomer, and identify its...
-
Identify the reagents you would use to achieve each of the following transformations: (a) (b) (c) (d) (e) OTs `CN
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


