Would you expect these two molecules to have nearly identical properties? H 2, H
Question:
Would you expect these two molecules to have nearly identical properties?
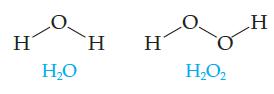
Transcribed Image Text:
Η Η Ο Ἡ H Η2Ο, H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
The molecules H 2 O water and H 2 O 2 hydrogen peroxide h...View the full answer

Answered By

Irfan Ali
I have a first class Accounting and Finance degree from a top university in the World. With 5+ years experience which spans mainly from the not for profit sector, I also have vast experience in preparing a full set of accounts for start-ups and small and medium-sized businesses. My name is Irfan Ali and I am seeking a wide range of opportunities ranging from bookkeeping, tax planning, business analysis, Content Writing, Statistic, Research Writing, financial accounting, and reporting.
4.70+
249+ Reviews
530+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
You have two samples of water, each made up of different isotopes of hydrogen: one contains and the other, a. Would you expect these two water samples to be chemically similar? b. Would you expect...
-
You have two identical containers, one containing gas A and the other gas B. The masses of these molecules arc m A = 3.34 X 10-27 kg and m B = 5.34 X 10-26 kg.Both gases arc under the same pressure...
-
Cyclopropane (C3H6) has the shape of a triangle in which a C atom is bonded to two H atoms and two other C atoms at each corner. Cubane (C8H8) has the shape of a cube in which a C atom is bonded to...
-
96. A 66-year-old woman with a long history of heavy smoking presents to her doctor with complaints of shortness of breath and chronic coughing that has been present for about 2 years and has been...
-
If you became the new manager at a restaurant with high employee turnover, what actions would you take to increase retention of employees?
-
Pro forma statements Provincial Imports, Inc., has assembled past (2015) financial statements (income statement and balance sheet below) and financial projections for use in preparing financial plans...
-
Describe what would happen if the learning rate ???? did not decline?
-
Shadow Corp. has no debt but can borrow at 8 percent. The firms WACC is currently 11 percent, and the tax rate is 35 percent. a. What is Shadows cost of equity? b. If the firm converts to 25 percent...
-
Glacier Products Inc. is a wholesaler of rock climbing gear. The company began operations on January 1, Year 1. The following transactions relate to securities acquired by Glacier Products Inc.,...
-
Work together to finish the following statement: You dont need Greek numerical prefixes when naming simple ionic compounds because . . .
-
Predict the formula of the compound that forms between phosphorus (P) and hydrogen.
-
Describe what makes a family business different from other types of business.
-
Refer to Figure 11.2: Is it more costly to build in Los Angeles or in Washington DC? What is the cost difference? Figure 11.2 Location Factors Costs shown in RSMeans Square Foot Costs are based on...
-
Suppose the prism in Figure P33.27 is immersed in a liquid in which the speed of light is lower than the speed of light in glass. Describe what happens to the light shown entering at normal...
-
Each year, the AICPA issues a general audit risk alert document and a number of industry audit risk alerts. If you can obtain access to a current copy of either the general alert or one of the...
-
The multieffect distillation system shown in Figure 11-4 appears to be able to cut energy use in half; however, the reduction is not this large. Explain why. Figure 11-4 F PL D, D Reflux B PH
-
Schemes 11-6E and 11-6F accomplish the same task of removing and purifying an intermediate component. a. What factors enter into the decision to use scheme \(11-6 \mathrm{~F}\) instead of \(11-6...
-
Plot the Lissajous figure defined by x = cos 2t, y = sin 7t, 0 t 2. Explain why this is a closed curve even though its graph does not look closed.
-
Which of the following streaming TV devices does not involve use of a remote controller? A) Google Chromecast B) Apple TV C) Amazon Fire TV D) Roku
-
a. What is the average time required for H 2 to travel 1.00 meter at 298 K and 1 atm? b. How much longer does it take N 2 to travel 1 m on average relative to H 2 under these same conditions? c....
-
As mentioned in Section 33.3, the only differences between the quantities mp , ave , and rms involve constants. a. Derive the expressions for ave and v rms relative to mp provided in the text....
-
At what temperature is the rms of Ar equal to that of SF 6 at 298 K? Perform the same calculation for mp .
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


