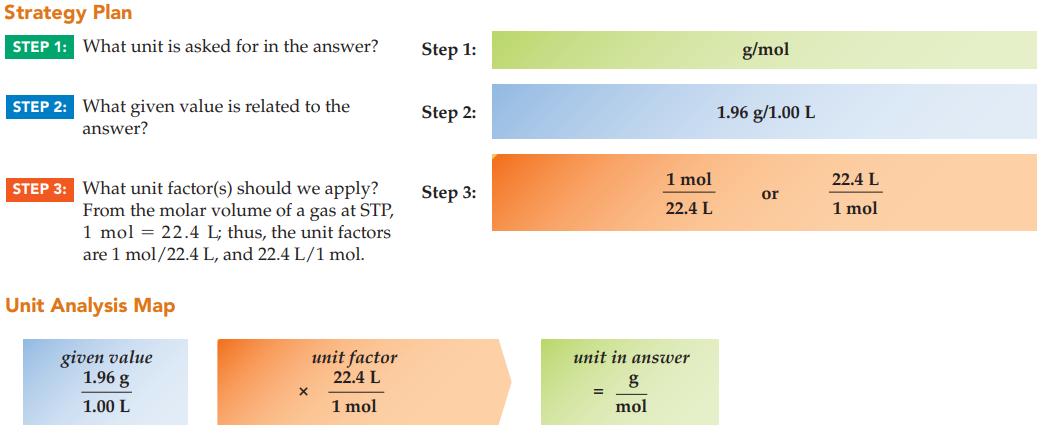
A fire extinguisher releases 1.96 g of an unknown gas that occupies 1.00 L at STP. What
Question:
A fire extinguisher releases 1.96 g of an unknown gas that occupies 1.00 L at STP. What is the molar mass (g/mol) of the unknown gas?
Transcribed Image Text:
Strategy Plan STEP 1: What unit is asked for in the answer? STEP 2: What given value is related to the answer? STEP 3: What unit factor(s) should we apply? From the molar volume of a gas at STP, 1 mol = 22.4 L; thus, the unit factors are 1 mol/22.4 L, and 22.4 L/1 mol. Unit Analysis Map given value 1.96 g 1.00 L X unit factor 22.4 L 1 mol Step 1: Step 2: Step 3: 1 mol 22.4 L unit in answer g mol = g/mol 1.96 g/1.00 L or 22.4 L 1 mol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
Because the unknown gas is from a fire extinguisher we suspect ...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Communication Ethics is how one uses language, media, journalism, and creates relationships guided by individual morals and values. This ethics assumes being aware of the consequences of behavior and...
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
If 48.3 g of an unknown gas occupies 10.0 L at 40C and 3.10 atm, what is the molar mass of the gas?
-
In Problem, find the indicated derivative. Find y (5) if d 2 y/dx 2 = 3 3x + 2.
-
Pas Company issued $1,000,000 of bonds on January 1, 2012. Instructions (a) Prepare the journal entry to record the issuance of the bonds if they are issued at (1) 100, (2) 98, and (3) 103. (b)...
-
a. Construct a frequency distribution table for blood glucose levels of male participants using the classes 7589, 90104, 105119, 120134, and 135149. b. Calculate the relative frequency and percentage...
-
Comparing Mortgage Sources. Using Personal Financial Planner Sheet 44 along with online or in-person research, obtain information from several mortgage companies and other financial institutions...
-
Hatfield Medical Supply's stock price had been lagging its industry averages, so its board of directors brought in a new CEO, Jaiden Lee. Lee had brought in Ashley Novak, a finance MBA who had been...
-
Based on the following data, and assuming Notes Payable is the only other item on the Balance Sheet, which of the following is correct? Contributed Capital $12,000 Total Assets $176,000 Accounts...
-
Boron trifluoride gas is used in the manufacture of computer chips. Given that 1.505 g of the gas occupies 497 mL at STP, what is the molar mass of boron fluoride gas?
-
A common name for fructose is fruit sugar. What is the molecular formula of fructose if the empirical formula is CH 2 O, and the approximate molar mass is 180 g/mol? (a) CHO (b) CH 2 O (c) CH 2 O 6...
-
Who executes the process and does it need to be them?
-
Use your own academic report, issued by your institute, as an example. Ask a database administrator how they use normalization steps to transform the details of the report into a set of relations in...
-
Francis Corp. has two divisions, Eastern and Western. The following information for the past year is for each division: Francis has established a hurdle rate of 9 percent. Required: 1. Compute each...
-
The enzyme lipase catalyzes the hydrolysis of esters of fatty acids. The hydrolysis of p-nitrophenyloctanoate was followed by measuring the appearance of p-nitrophenol in the reaction mixture: The...
-
Use values of r cov (Table 17.1) to estimate the XY bond lengths of ClF, BrF, BrCl, ICl and IBr. Compare the answers with values in Fig. 17.8 and Table 17.3, and comment on the validity of the method...
-
From a square whose side has length \(x\), measured in meters, create a new square whose side is \(10 \mathrm{~m}\) longer. Find an expression for the sum of the areas of the two squares as a...
-
(a) Show that for the orbital-angular-momentum eigenfunctions, the smallest possible value for the angle between L and the z axis obeys the relation cos2 = l/ (l + 1). (b) As l increases, does this...
-
Draw the major product for each of the following reactions: (a) (b) (c) 1) 9-BBN 2) H2O2, NaOH 1) Disiamylborane 2) H20, NaOH
-
Lower-of-Cost-or-Market Fiedler Co. follows the practice of valuing its inventory at the lower-of-cost-or-market. The following information is available from the company's inventory records as of...
-
Conventional and Dollar-Value LIFO Retail As of January 1, 2010, Aristotle Inc. installed the retail method of accounting for its merchandise inventory. To prepare the store's financial statements at...
-
Retail, LIFO Retail, and Inventory Shortage Late in 2007, Joan Seceda and four other investors took the chain of Becker Department Stores private, and the company has just completed its third year of...
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


