According to general trends in the periodic table, predict which element in each of the following pairs
Question:
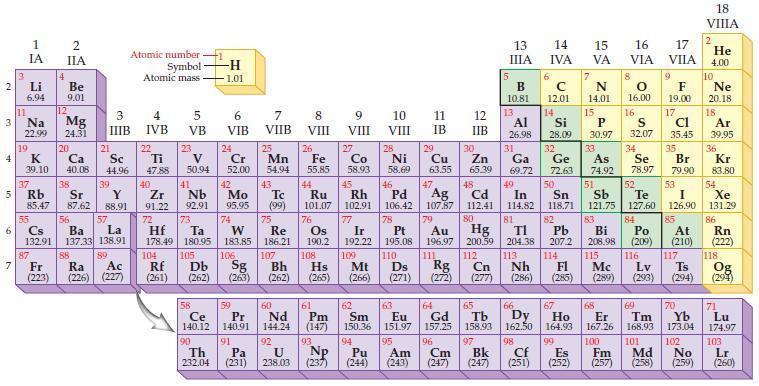
According to general trends in the periodic table, predict which element in each of the following pairs has the larger atomic radius.
(a) Rb or Sr
(b) As or Se
(c) Pb or Bi
(d) I or Xe.
Periodic Table:
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 117 116 Mc Ts Lv (289) (293) (294) 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
The atomic radius generally decreases across a period from left to right and increases down a group ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
According to general trends in the periodic table, predict which element in each of the following pairs has the larger atomic radius. (a) Li or Na (b) N or P (c) Mg or Ca (d) Ar or Kr. Periodic...
-
According to general trends in the periodic table, predict which element in each of the following pairs has the greater metallic character. (a) B or Al (b) Na or K (c) Mg or Ba (d) H or Fe. Periodic...
-
According to general trends in the periodic table, predict which element in each of the following pairs has the greater metallic character. (a) K or Ca (b) Mg or Al (c) Fe or Cu (d) S or Ar. Periodic...
-
A particle is thrown vertically upwards into the air. Its height s (in m) above the ground after time t (in seconds) is given by s = 25t 5t 2 (a) What height does the particle reach? (b)What is its...
-
Describe the advantages of performance budgeting and program budgeting over incremental budgeting in a governmental entity.
-
The forces on a dancer can be measured directly when a dancer performs a jump on a force plate that measures the force between her feet and the ground. A graph of force versus time throughout a...
-
Describe and give the advantages and disadvantages of (a) moving averages and (b) exponential smoothing. LO.1
-
During the current year, Tina purchases a beachfront condominium for $600,000, paying $150,000 down and taking out a $450,000 mortgage, secured by the property. At the time of the purchase, the...
-
eBook Show Me How Missing Amounts from Balance Sheet and Income Statement Data One item is omitted in each of the following summaries of balance sheet and income statement data for the following four...
-
Predict the missing value (?) for radioactive francium (Fr). The atomic radius, density, and melting point are given for elements in Group IA/1. Element Atomic Radius 248 pm 266 pm (?) pm Rb Cs Fr...
-
According to the general trend, the atomic radius (increases/decreases) proceeding from left to right in the periodic table. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10...
-
Sam Inc. leased a photocopier on January 1, 2019, for a three-year period. Payments, which are first due on the commencement date, are $3,000 per year. The $3,000 is comprised of $2,500 for the...
-
Management is what tradition used to call a liberal art: "liberal" because it deals with the fundamentals of knowledge, self-knowledge, wisdom, and leadership; "art" because it is a practice and...
-
Draft a five hundred and twenty five- to seven hundred-word internal communication planthat appropriately details your proposed solution to the internal team at CVS PHARMACY. In your communication...
-
Christopher Awnings was founded by Christopher Aminim in the early days of the retirement boom in the Okanagan to build and install custom retractable awnings for retirees to keep the sun out of the...
-
Leaders are responsible for making decisions that have long-term ramifications; thus, making the appropriate decisions can be stressful and leaders' decisions may vary. They often enhance employee...
-
Employee longevity A large insurance company has developed a model to identify the factors associated with employee turnover. The dependent variable is number of years an employee stays with the...
-
In what courts are disputes between the IRS and a taxpayer heard?
-
To balance the chemical equation SiH3 + O2 SiO2 + HO, you could introduce coefficients a, b, c, d and write aSiH3 + bO2 cSiO + dHO then write linear equations for each element. The equation for Si...
-
What is service cost, and what is the basis of its measurement?
-
In computing the interest component of pension expense, what interest rates may be used?
-
Explain the difference between service cost and prior service cost.
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


