According to general trends in the periodic table, predict which element in each of the following pairs
Question:
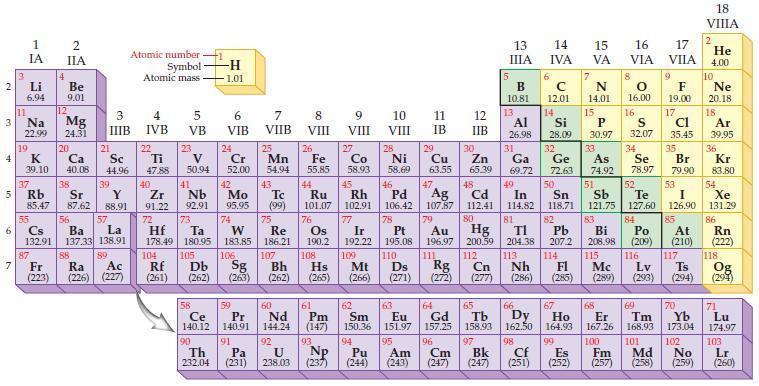
According to general trends in the periodic table, predict which element in each of the following pairs has the greater metallic character.
(a) B or Al
(b) Na or K
(c) Mg or Ba
(d) H or Fe.
Periodic Table:
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 116 Mc Ts Lv (289) (293) (294) 117 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
Metallic character generally increases from right to left across a period and from top to bottom wit...View the full answer

Answered By

HUSNA MOAB
In my hone town my just a home tutors for +2 students,for some time.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
According to general trends in the periodic table, predict which element in each of the following pairs has the greater metallic character. (a) K or Ca (b) Mg or Al (c) Fe or Cu (d) S or Ar. Periodic...
-
Based on the general trends in the periodic table, predict which element in each of the following pairs has the smaller atomic radius: (a) Na or K (b) P or N (c) Ca or Ni (d) Si or S. Periodic Table:...
-
According to general trends in the periodic table, predict which element in each of the following pairs has the larger atomic radius. (a) Li or Na (b) N or P (c) Mg or Ca (d) Ar or Kr. Periodic...
-
Find the turning points on the curve y = 2x 3 5x 2 + 4x 1 and determine their nature. Find the point of inflection and sketch the graph of the curve.
-
Explain how strategic planning, Budgeting, and performance measurement can be integrated in a government and why this integration is desirable.
-
A person wearing these shoes stands on a smooth, horizontal rock. She pushes against the ground to begin running. What is the maximum horizontal acceleration she can have without slipping? (a) 0.20g;...
-
What is meant by the term deseasonalized demand? LO.1
-
Unter Components manufactures low-cost navigation systems for installation in ride-sharing cars. It sells these systems to various car services that can customize them for their locale and business...
-
Sanyu Sony started a new business and completed these transactions during December. Dec. 1 Sanyu Sony transferred $69,600 cash from a personal savings account to a checking account in the name of...
-
According to general trends in the periodic table, predict which element in each of the following pairs has the larger atomic radius. (a) Rb or Sr (b) As or Se (c) Pb or Bi (d) I or Xe. Periodic...
-
According to the general trend, the atomic radius (increases/decreases) proceeding from left to right in the periodic table. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10...
-
The ordinary share price of Chock-stock plc is 2.00 per share and the company has been paying a dividend of 30p per share for 10 years. The company plans to retain the next three years dividends to...
-
Investigate the Mercedes Benz company and you have to cover this topic " For Business prospects, Market growth, Market quality, and Environmental aspects are three most important factors. Explain...
-
The case study for Goodwill Industries and how they "do good" as a core business strategy. What are Goodwill's competitive advantages? Goodwill has found success in social services. What problems...
-
Cosmic Cals (Pty) Ltd , a seller of personalized scientific calculators, had an inventory of 40 calculators. The value of these calculators is R15 400 each on the 1 January 2022. During the current...
-
Perform an analysis of Best Buy Co. Inc. Your analysis will draw on the Form 10K (as of February 2013). Your analysis can include information prior to February 2013 but should not draw on any...
-
Research organizational structure of a company of your choice. Use your understanding of organizational structure to analyze whether this organization's structure is the best choice for the business...
-
What is the legislative process concerning tax laws? Where does tax legislation often begin?
-
Explain the operation of the dividends received deduction.
-
Given the items and amounts shown on page 1094, compute the actual return on plan assets: fair value of plan assets at the beginning of the period $9,500,000; benefits paid during the period...
-
How does an asset gain or loss develop in pension accounting? How does a liability gain or loss develop in pension accounting?
-
What is the meaning of corridor amortization?
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...
Stardust To Planets A Geological Tour Of The Solar System 1st Edition - ISBN: 0312131887 - Free Book

Study smarter with the SolutionInn App


