According to general trends in the periodic table, predict which element in each of the following pairs
Question:
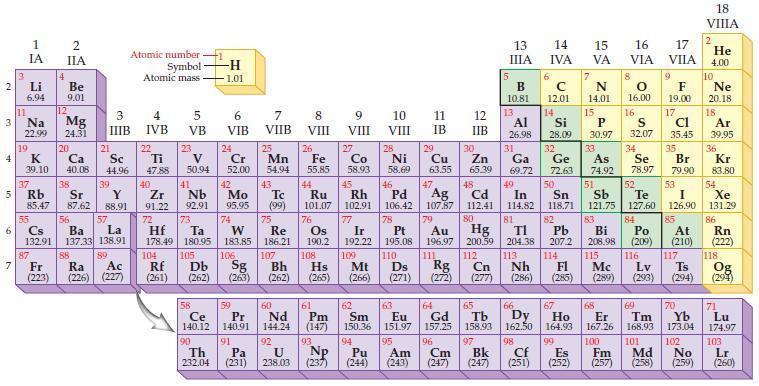
According to general trends in the periodic table, predict which element in each of the following pairs has the larger atomic radius.
(a) Li or Na
(b) N or P
(c) Mg or Ca
(d) Ar or Kr.
Periodic Table:
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 117 116 Mc Ts Lv (289) (293) (294) 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Predictions based on general trends Pair Element with larger ...View the full answer

Answered By

Divya Munir
I hold M.Sc and M.Phil degrees in mathematics from CCS University, India and also have a MS degree in information management from Asian institute of technology, Bangkok, Thailand. I have worked at a international school in Bangkok as a IT teacher. Presently, I am working from home as a online Math/Statistics tutor. I have more than 10 years of online tutoring experience. My students have always excelled in their studies.
4.90+
0 Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
According to general trends in the periodic table, predict which element in each of the following pairs has the larger atomic radius. (a) Rb or Sr (b) As or Se (c) Pb or Bi (d) I or Xe. Periodic...
-
According to general trends in the periodic table, predict which element in each of the following pairs has the greater metallic character. (a) B or Al (b) Na or K (c) Mg or Ba (d) H or Fe. Periodic...
-
According to general trends in the periodic table, predict which element in each of the following pairs has the greater metallic character. (a) K or Ca (b) Mg or Al (c) Fe or Cu (d) S or Ar. Periodic...
-
Consider the integral where n is an integer. Using the trigonometric identity 1 + tan 2 x = sec 2 x, show that and hence obtain the recurrence relation Use this to find (Recurrence relations of this...
-
What is the difference between two types of line-item budgeting approachesincremental budgeting and zero-based budgeting? Which of the two approaches is more widely used by governments?
-
For a person wearing these shoes, whats the maximum angle (with respect to the horizontal) of a smooth rock that can be walked on without slipping? (a) 42; (b) 50; (c) 64; (d) larger than 90.
-
What is a seasonal index? How is it calculated? LO.1
-
Clothes, Inc., has an average annual demand for red, medium polo shirts of 25,000 units. The cost of placing an order is $80 and the cost of carrying one unit in inventory for one year is $25....
-
Betty's Bakery has the following standard cost sheet for one unit of its most popular cake: Direct materials Direct labor SO SP 1.2 pounds $ 1.50 per pound 0.8 houra $12.00 per hour During the month...
-
Predict the missing value (?) for radioactive francium (Fr). The atomic radius, density, and melting point are given for elements in Group IA/1. Element Atomic Radius 248 pm 266 pm (?) pm Rb Cs Fr...
-
According to the general trend, the atomic radius (increases/decreases) proceeding from left to right in the periodic table. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10...
-
How effective is private life insurance in meeting the problem of premature death? LO3
-
From the perspective of organizational structure, design, and control, what have gone wrong at PNB? What factors have contributed to its current state of disarray? What should be done next to help...
-
Your goal is to advise the President on domestic economic policy. Role You are the chair of the Council of Economic Advisors (CEA) Audience Your audience is the President of the United States....
-
omework quiz 2.1 #1 stem plot The miles per gallon rating for 30 cars are shown below (lowest to highest). 19, 19, 19, 20, 21, 21, 25, 25, 25, 26, 26, 28, 29, 31, 31, 32, 32, 33, 34, 35, 36, 37, 37,...
-
Your answers are saved automatically. Question Completion Status: QUESTION 1 13 points Save Answer Library A computer memory manufacturer specifies that its memory chip stores data incorrectly an...
-
Analyze the possible reasons for and responses to Chung's request for a private office. What factors might impact Leary's decision? Identify at least two challenges and dilemmas in managing...
-
What is administrative authority, and who publishes it?
-
Which of the followingcarbocations is the least stable? CH3CH2 . CH3CHCH3 CH3 I . CH3C0 T CH3 IV. V. CH3 CH3CCH2 CH3
-
What is meant by prior service cost? When is prior service cost recognized as pension expense?
-
What are liability gains and losses, and how are they accounted for?
-
If pension expense recognized in a period exceeds the current amount funded by the employer, what kind of account arises, and how should it be reported in the financial statements? If the reverse...
-
(15 points) Stressed $2.500,000 of S% 20 year bands. These bonds were issued Jary 1, 2017 and pay interest annually on each January 1. The bonds yield 3% and was issued at $325 8S! Required (2)...
-
Packaging Solutions Corporation manufactures and sells a wide variety of packaging products. Performance reports are prepared monthly for each department. The planning budget and flexible budget for...
-
1. A company issued 10%, 10-year bonds with a par value of $1,000,000 on January 1, at a selling price of $885,295 when the annual market interest rate was 12%. The company uses the effective...

Study smarter with the SolutionInn App


