According to the general trend, metallic character (increases/decreases) proceeding up a group of elements in the periodic
Question:
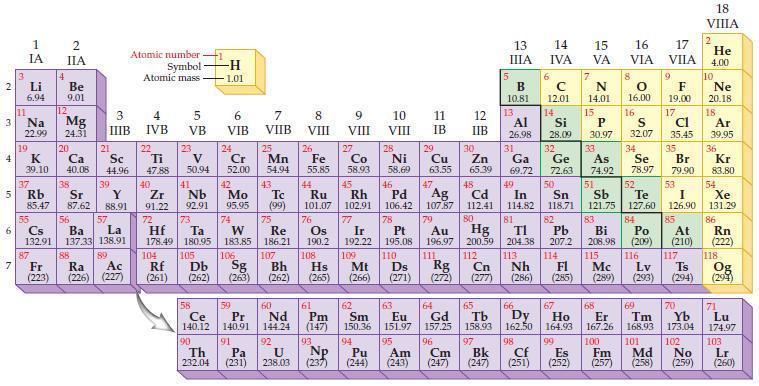
According to the general trend, metallic character (increases/decreases) proceeding up a group of elements in the periodic table.
Periodic Table:
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 117 116 Mc Ts Lv (289) (293) (294) 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Metallic character is increased from top to bottom down the ...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
According to the general trend, the atomic radius (increases/decreases) proceeding up a group of elements in the periodic table. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K...
-
Which statement is true about trends in metallic character? a) Metallic character increases as you move to the right across a row in the periodic table and increases as you move down a column. b)...
-
According to the general trend, the ionization energy for a group of elements (increases/decreases) proceeding up a group in the periodic table. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA Na...
-
The United Arab Emirates (UAE) is unique in that expatriates constitute more than 80 per cent of the population. As the country continues to grow and accept foreigners at an astounding pace, Emiratis...
-
Continuing Professional Education. As part of your audit firms quality control policies, it maintains a record of continuing profession education (CPE) taken by professional staff members. Following...
-
A 60.0-m-long brass rod is struck at one end. A person at the other end hears two sounds as a result of two longitudinal waves, one traveling in the metal rod and the other traveling in air. What is...
-
What action should be taken when unacceptable error is found in tracking a forecast? LO.1
-
You are the audit senior for the 2015 yearend audit of Vision Quest (VQ), a publicly traded Canadian company that is one of the largest and fastest online vision care providers in the world. Because...
-
The Holtz Corporation acquired 80 percent of the 100,000 outstanding voting shares of Devine, Inc., for $7.20 per share on January 1, 2020. The remaining 20 percent of Devine's shares also traded...
-
According to the general trend, metallic character (increases/decreases) proceeding from left to right in the periodic table. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K...
-
Refer to the periodic table and select the symbol of the element that fits each of the following descriptions. (a) The fourthperiod alkali metal (b) The fourthperiod alkaline earth metal (c) The rare...
-
Using the information provided in E18-15, assume now that Stewart Standard and Kane Kite are IFRS reporters. Required a. Determine the lessors classification of the lease. b. Measure the right-of-use...
-
Joint Ventures are a common Mode of Entry in international business. Appreciate if in-depth elaboration provided on its advantages and disadvantages. Also briefly mention the factors which make joint...
-
The field excursion is intended to give students an opportunity to carry out an applied geographical research project based on observation, data recording, and analysis. Using a field site of your...
-
An angry coworker is expressing their needs through a rush of emotion and snide comments while another coworker is trying to interpret them to provide some help and support. You are a manager and...
-
You may have a general understanding of the difference between ethics and legality , but could you explain the distinction? It is not always easy to know where to draw the line between the two. Some...
-
Someone can be a good leader but not be a very good manager and vice-versa. Leadership is creating a vision for others to follow, establishing corporate values and ethics, and transforming the way...
-
What is the most common way taxpayers pay their income tax liability during the year?
-
In the simple quantity theory of money, what will lead to an increase in aggregate demand? In monetarism, what will lead to an increase in aggregate demand?
-
Why didnt the FASB cover both types of postretirement benefitspensions and healthcarein the earlier pension accounting rules?
-
What are the major differences between postretirement healthcare benefits and pension benefits?
-
What is the difference between the APBO and the EPBO? What are the components of postretirement expense?
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


