According to the general trend, the atomic radius (increases/decreases) proceeding up a group of elements in the
Question:
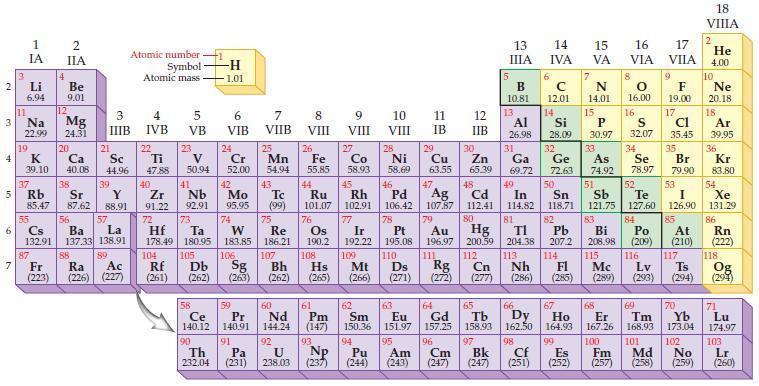
According to the general trend, the atomic radius (increases/decreases) proceeding up a group of elements in the periodic table.
Periodic Table:
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 117 116 Mc Ts Lv (289) (293) (294) 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
Atomic radius is increased from top to bottom d...View the full answer

Answered By

Michael Owens
I am a competent Software Engineer with sufficient experience in web applications development using the following programming languages:-
HTML5, CSS3, PHP, JAVASCRIPT, TYPESCRIPT AND SQL.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
According to the general trend, metallic character (increases/decreases) proceeding up a group of elements in the periodic table. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K...
-
According to the general trend, the ionization energy for a group of elements (increases/decreases) proceeding up a group in the periodic table. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA Na...
-
The two employees had an altercation on the job site, one used a blunt instrument to inflict a wound on the other employee. the company terminated one employee and suspended the other. The Union...
-
Prepare journal entries to record each of the following sales transactions of TFC Merchandising. TFC uses a perpetual inventory system and the gross method. May 1 9 Sold merchandise for $600, with...
-
GAO Independence Standards. Indicate which of the following activities performed by an auditor for a governmental client are (a) Allowable, (b) Permitted if safeguards are in place, or (c)...
-
His body is again leaning back at 30.0 o to the vertical, but now the height at which the rope is held above but still parallel to the ground is varied. The tension in the rope in front of the...
-
What is random variation? LO.1
-
Assume the same information as E14-9 and that Steffi Graf Inc. reports net income in 2008 of $120,000 and in 2009 of $140,000. Total holding gains (including any realized holding gain or loss)...
-
13 Exercise 22-22C Assigning joint product costs LO C3 5 points Pirate Seafood Company purchases lobsters and processes them into tails and flakes. It sells the lobster tails for $20.20 per pound and...
-
According to the general trend, the atomic radius (increases/decreases) proceeding from left to right in the periodic table. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10...
-
According to the general trend, metallic character (increases/decreases) proceeding from left to right in the periodic table. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K...
-
The Minoso Corporation anticipates a 20 percent increase in sales for 2014, 2015, and 2016. Minoso is currently operating at full capacity and thus expects to increase its investment in both current...
-
Q2 2 Points The time between students pinging professor with questions during an exam is modeled by an exponential random variable X (measured in minutes) with parameter (usual notation) Q2.1 1 Point...
-
A. Describe what the formula P = M A E represents. B. What happens if one of these factors becomes deficient? C. In terms of performance, identify the four different types of reinforcement. Provide...
-
1-3.2) K Question 10, 3.1.37 Part 1 of 6 > HW Score: 53.33%, 6.4 of 12 points O Points: 0 of 1 Save Because the mean is very sensitive to extreme values, it is not a resistant measure of center. By...
-
Research the company and obtain the following information: Mission Statement - Purpose of their existence Goals and objectives (What are they in business for) SWOT analysis for this company Based on...
-
please write one page for the concept of organizational structure one page paper of factors affecting organizational structure.
-
Sheniqua, a single taxpayer, had taxable income of $75,053. Her employer withheld $14,290 in federal income taxes from her paychecks throughout the year. Using the tax tables, would Sheniqua receive...
-
Reduction in sales All of the above 29. Belt of an electric motor is broken, it needs a. Corrective maintenance b. Scheduled maintenance c. Preventive maintenance d. Timely maintenance. 30. The...
-
A headline in the Wall Street Journal stated, Firms Increasingly Tap Their Pension Funds to Use Excess Assets. What is the accounting issue related to the use of these excess assets by companies?
-
Where can authoritative iGAAP related to the accounting for pensions be found?
-
Briefly describe some of the similarities and differences between U.S. GAAP and iGAAP with respect to the accounting for pensions.
-
Discuss American History
-
Your firm has developed a new lithium ion battery polymer that could enhance the performance of lithion ion batteries. These batteries have applications in many markets including cellphones, laptops,...
-
Need help analyzing statistical data 1. ANOVA) True or false: If we assume a 95% confidence level, there is a significant difference in performance generally across all groups. 2. (t-test) True or...

Study smarter with the SolutionInn App


