According to the general trend, the ionization energy for a period of elements (increases/decreases) proceeding from left
Question:
According to the general trend, the ionization energy for a period of elements (increases/decreases) proceeding from left to right in the periodic table.
Periodic Table:
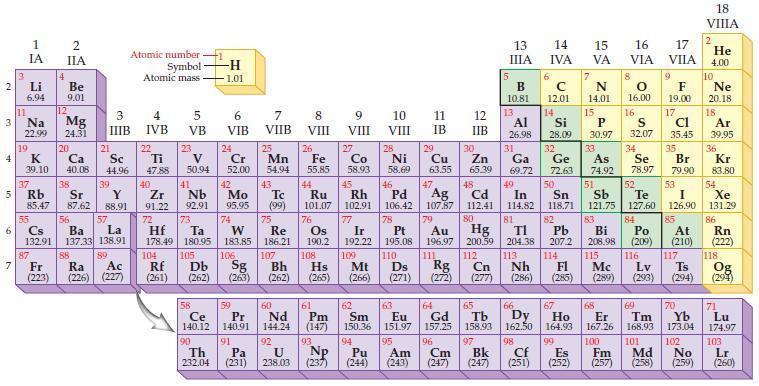
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 117 116 Mc Ts Lv (289) (293) (294) 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The ionization energy for a period of elements increases proceeding from left to right in the period...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
The financial information provided below is for two companies which operate in similar retail fields, using the same business and accounting policies A. Calculate for each company, ratios that shows...
-
According to the general trend, the ionization energy for a group of elements (increases/decreases) proceeding up a group in the periodic table. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA Na...
-
The accompanying graphs show the first ionization energies and electron affinities of the period 3 elements. Refer to the graphs to answer the questions that follow. a. Describe the general trend in...
-
Sulfur trioxide reacts with water to form sulfuric acid, a major contributor to acid rain. One origin of SO 3 is the combustion of sulfur, which is present in small quantities in coal, according to...
-
Which standard-setting bodies have responsibility for establishing accounting and reporting standards for (1) state and local governments, (2) business organizations. (3) not-for-profit...
-
Stenberg plc is preparing its financial statements for the year ended 30 November 2018. On 1 May 2018, the company purchased a factory for the manufacture of optical disks, paying 24,000,000. The...
-
20-5. Cules son las seis etapas del proceso de venta personal?
-
1. What are the biggest challenges to long-term success and profitability for Loblaws, Sobeys, and Metro? 2. The three main players in the Canadian grocery market each operate under various brands....
-
Just tell me what R code to use here for questions. If you don't need R, then at least tell me how to do them. Estimate the relationship between corn demand and price using time series data from 1926...
-
State the number of valence electrons for each of the following elements. (a) He (b) Pb (c) Se (d) Ne (e) Cs (f) Ga (g) Sb (h) Br.
-
State the number of valence electrons for each of the following elements. (a) H (b) B (c) N (d) F (e) Ca (f) Si (g) O (h) Ar.
-
Automobile traffic passes a point P on a road of width w feet at an average rate of R vehicles per second. Although the arrival of automobiles is irregular, traffic engineers have found that the...
-
Use as many directional terms as possible to describe therelationshipbetween: a. the antecubital region and the poplitealregion b. the acromial region and the mentalregion c. the gluteal region and...
-
Average rate of return-cost savings Maui Fabricators Inc. is considering an investment in equipment that will replace direct labor. The equipment has a cost of $114,000 with a $10,000 residual value...
-
Alan was rated as excellent on his individual work performance evaluation, earning him $2,000, provided as a merit pay increase. His annual salary this year is $48,000. He works in a team of 3...
-
Josie spends $60 at the end of each month on cigarettes. If shestops smoking and invests the same amount in an investment planpaying 6% compounded monthly, how much will she have after fiveyears? 2...
-
Say we have a Boeing 747 whose longitudinal flight dynamics for a given flight condition may be approximated using the following state equation (uncontrolled motion; thus no need to consider the...
-
What is boot? How does the receipt of boot affect a like-kind exchange?
-
Using Gauss-Jordan elimination, invert this matrix ONLY 0 0 0 0 1
-
In 2010, Amir ante Corporation had pretax financial income of $168,000 and taxable income of $120,000. The difference is due to the use of different depreciation methods for tax and accounting...
-
Oxford Corporation began operations in 2010 and reported pretax financial income of $225,000 for the year. Oxfords tax depreciation exceeded its book depreciation by $40,000. Oxfords tax rate for...
-
Using the information from BE19-2, assume this is the only difference between Oxfords pretax financial income and taxable income. Prepare the journal entry to record the income tax expense, deferred...
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...
-
***Please answer the following using excel and showcasing the formulas/calculations used*** thank you so much Financial information on AAA Ltd. is shown below. AAA Ltd. Income Statement For the Year...
-
2. In an account Anh Paglinawan currently has $216,670.00. At a rate of 8.00% how long will it take for them to have $298,390.00 assuming semi-annually compounding? (Hint: compute the exact years, do...

Study smarter with the SolutionInn App


