Sulfur trioxide reacts with water to form sulfuric acid, a major contributor to acid rain. One origin
Question:
Sulfur trioxide reacts with water to form sulfuric acid, a major contributor to acid rain. One origin of SO3 is the combustion of sulfur, which is present in small quantities in coal, according to the following equation:
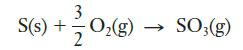
Given the thermochemical information below, determine the heat of reaction for this process:
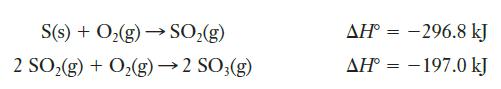
Strategy We need to use the reactions whose enthalpy changes are known to construct a pathway that results in the desired reaction. In this case, we can see that SO2 is formed in the first reaction we are given and then is consumed in the second. It is critical to pay attention to the precise stoichiometry of the given and desired reactions. The reaction of interest produces just 1 mole of SO3, whereas the second reaction we are given produces 2 moles. So we will need to correct for that.
Step by Step Answer:

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme





