Calculate the enthalpy change when 245 g of ice melts. Strategy The H fus value in Table
Question:
Calculate the enthalpy change when 245 g of ice melts.
Strategy The ΔHfus value in Table 9.3 is in J/mol, so the amount of ice must be converted into moles. Multiplying the number of moles by ΔHfus will provide the desired quantity.
Table 9.3
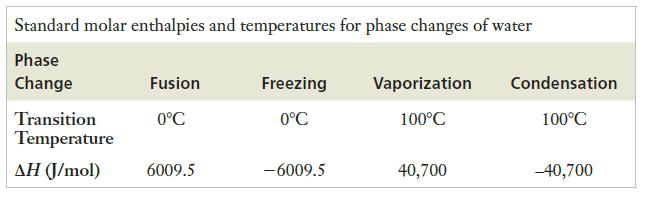
Transcribed Image Text:
Standard molar enthalpies and temperatures for phase changes of water Phase Change Transition Temperature AH (J/mol) Fusion 0°C 6009.5 Freezing 0°C -6009.5 Vaporization 100°C 40,700 Condensation 100°C -40,700
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Analyze Your Answer The enthalpy of fusion is constant at 6 kJmol so the enthalpy change depends on ...View the full answer

Answered By

Alexandra Scotg
My first teaching job came in 2014- I worked at a further education college, teaching people from ages 16 onwards. Then in 2017- after having my daughter, I needed a flexible job. I started my online teaching journey and 3 years later I still love it!
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
The enthalpy change when liquid methanol, CH3OH, vaporizes at 25oC is 38.0 kJ/mol. What is the entropy change when 1.00 mol of vapor in equilibrium with liquid condenses to liquid at 25oC? The...
-
We use the formula PV = FV (1/1+R)^N to calculate Present Value. The formula is used to figure out how much a future sum is worth today, given that there is always a discount rate. In class, we...
-
When 15.3 g of sodium nitrate, NaNO3, was dissolved in water in a calorimeter, the temperature fell from 25.00oC to 21.56oC. If the heat capacity of the solution and the calorimeter is 1071 J/oC,...
-
How is the standard form of a circles equation obtained from its general form?
-
In each case below, accounting conventions may have been violated. 1. After careful study, Lipski Company, which has offices in 40 states, has determined that its method of depreciating office...
-
The publishing company recently came up with some additional data for the 15 books in the original sample. Two new variables, production expenditure (x5) and number of prepublication reviewers (x6),...
-
Do you believe your country should adopt a national industrial policy? Why or why not? LO.1
-
Exhibit 4.21 presents selected operating data for three retailers for a recent year. Macys operates several department store chains selling consumer products such as brand-name clothing, china,...
-
Fingen's 12-year, $1,000 par value bonds pay 14 percent interest annually. The market price of the bonds is $880 and the market's required yield to maturity on a comparable-risk bond is 18 percent....
-
Sulfur trioxide reacts with water to form sulfuric acid, a major contributor to acid rain. One origin of SO 3 is the combustion of sulfur, which is present in small quantities in coal, according to...
-
Write an SQL query that would ask the database to count the number of different types of t-shirts available and total their price. Your output should show only the field names: NumberOfTshirts...
-
Haas Company is a retail company that specializes in selling outdoor camping equipment. The company is considering opening a new store on October 1, 2015. The company president formed a planning...
-
1. Using appropriate examples, compare and contrast the genetic diversity of marine fish species with freshwater fish species (8 marks) 2. Your class went on a trip and discovered a crater lake on...
-
Find sin(29) given that cos(0) = and 0
-
Amazing Aquariums began as a class project on new business development. Now that the visionaries behind the idea have graduated, they want to explore their business idea and see if the concept could...
-
3. Modify the program of Example 05 so that, it takes and prints values using the following two functions respectively. void get (double *&a, int& n); void print (double *a, int n); 4. Following is a...
-
A company will be financing its operations with and a capital budget is P40,000,000 and a debt-to-equity ratio of 1. The interest rate on company's debt is 10%. The expected return on equity by the...
-
Goldstein Mines paid $424,000 for the right to extract ore from a 200,000-ton mineral deposit. In addition to the purchase price, Goldstein Mines also paid a $100 filing fee, a $1,900 license fee to...
-
In a paragraph of approximately 150-200 words, analyze a film or TV/Streaming Show poster of your choosing by focusing on the ways in which representations in the poster are gendered. Include an...
-
Discuss how the DebyeHckel screening length changes as the (a) Temperature (b) Dielectric constant, (c) Ionic strength of an electrolyte solution are increased.
-
Why is it not possible to measure the Gibbs energy of solvation of Cl directly?
-
Why are activity coefficients calculated using the DebyeHckel limiting law always less than one?
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer
-
Domino is 4 0 years old and is married out of community of property with the exclusion of the accrual system to Dolly ( 3 5 ) . They have one child, Domonique, who is 1 1 years old. Domino resigned...
-
YOU ARE CREATING AN INVESTMENT POLICY STATEMENT FOR JANE DOE General: 60 years old, 3 grown children that are living on their own and supporting themselves. She is in a very low tax rate so we don't...

Study smarter with the SolutionInn App


