Calculate the mass in grams for a single molecule of carbon dioxide, CO 2 (given that MM
Question:
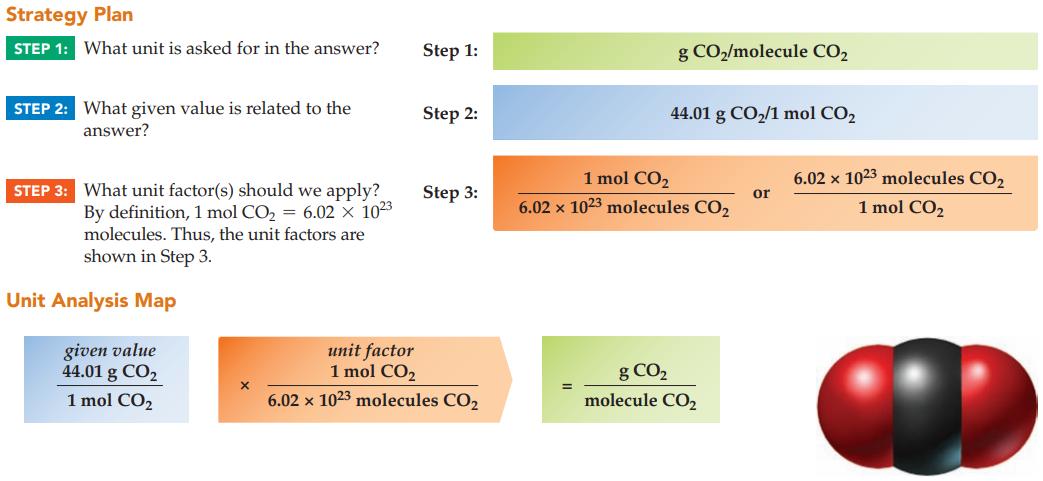
Calculate the mass in grams for a single molecule of carbon dioxide, CO2 (given that MM = 44.01 g/mol).
Transcribed Image Text:
Strategy Plan STEP 1: What unit is asked for in the answer? STEP 2: What given value is related to the answer? STEP 3: What unit factor(s) should we apply? By definition, 1 mol CO₂ = 6.02 x 10²3 molecules. Thus, the unit factors are shown in Step 3. Unit Analysis Map given value 44.01 g CO₂ 1 mol CO₂ Step 1: Step 2: Step 3: unit factor 1 mol CO₂ 6.02 x 1023 molecules CO₂ g CO₂/molecule CO₂ 44.01 g CO₂/1 mol CO₂ 1 mol CO₂ 6.02 x 1023 molecules CO2 g CO₂ molecule CO₂ or 6.02 x 1023 molecules CO₂ 1 mol CO₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
We apply the unit factor 1 mo...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Calculate the mass in grams for a single molecule of carbon monoxide, CO.
-
Calculate the mass in grams of the following. a. 0.205 mol Fe b. 0.79 mol F c. 5.8 mol CO2 d. 48.1 mol K2CrO4
-
Write a for loop to populate vector userGuesses with NUM GUESSES integers. and user enters 9 5 2, then userGuesses is (9, 5, 2). Learn how our autograder works 1 #include 2 #include 3 using namespace...
-
Consider a spherical fluid particle in an inviscid fluid (no shear stresses). If pressure and gravitational forces are the only forces acting on the particle, can they cause the particle to rotate?...
-
For the year ended December 31, 2012, Radar Company reports the following summary payroll data. Radar Company?s payroll taxes are: FICA 8%, state unemployment 2.5% (due to a stable employment...
-
Voyles Corporation, a calendar year taxpayer formed five years ago, desires to make an Selection beginning in 2019. Sue and Andrea each own one-half of the Voyles stock. a. How does Voyles make the...
-
How do companies report foreign currency derivatives, such as forward contracts and options, on the balance sheet? LO9
-
The partnership agreement of ABC Associates provides that income should be allocated in the following manner: 1. Each partner receives interest of 20% of beginning capital. 2. Sue receives a salary...
-
IFRS vs. GAAP: Highlight key differences between International Financial Reporting Standards (IFRS) and Generally Accepted Accounting Principles (GAAP), and discuss the implications for multinational...
-
If 0.500 mol of silver combines with 0.250 mol of sulfur, what is the empirical formula of the silver sulfide product? (a) AgS (b) Ag 2 S (c) AgS 2 (d) Ag 5 S 5 (e) None of the above.
-
What is the mass of Avogadros number of ozone, O 3 , molecules?
-
A company that makes LED light bulbs tests 500 bulbs each day for defects. The number of defective bulbs, along with the proportion of defective bulbs for 20 days, is shown in the following table....
-
The amounts of caffeine in a sample of five-ounce servings of brewed coffee are shown in the histogram. Number of 5-ounce servings S 25- 20 15 10 25 12 10 1 2 70.5 92.5 114.5 136.5 158.5 Caffeine (in...
-
Tom, David, Dale, and Murdock are four business students who want to rent a four- bedroom apartment together for the fall semester. They have identified the three factors important to them in...
-
Listed below, out of order, are the steps in an accounting cycle. 1. Prepare the unadjusted trial balance. 2. Post journal entries to general ledger accounts. 3. Analyze transactions from source...
-
Consider Quick Start QFD Matrix 2 above. Which two technical specifications are strongly correlated with each other? Quick Start QFD Matrix 2 Strong positive correlation Some positive correlation ==...
-
A cylindrical solenoid of length \(\ell\) and radius \(R\) has \(n\) windings per unit length and carries a current \(I\). (a) Use the inductance expression \(L=\left(\mu_{0} N^{2} A ight) / \ell\)...
-
In applying the Numerov method to count the nodes in r, we assumed that changes sign as it goes through a node. However, there are functions that do not have opposite signs on each side of a node....
-
A manufacturer can sell product 1 at a profit of $20 per unit and product 2 at a profit of $40 per unit. Three units of raw material are needed to manufacture one unit of product 1, and six units of...
-
Classification of Costs and Interest Capitalization On January 1, 2010, Blair Corporation purchased for $500,000 a tract of land (site number 101) with a building. Blair paid a real estate brokers...
-
Interest during Construction Grieg Landscaping began construction of a new plant on December 1, 2010. On this date the company purchased a parcel of land for $139,000 in cash. In addition, it paid...
-
Capitalization of Interest Laserwords Inc. is a book distributor that had been operating in its original facility since 1985. The increase in certification programs and continuing education...
-
An underlying asset price is at 100, its annual volatility is 25% and the risk free interest rate is 5%. A European call option has a strike of 85 and a maturity of 40 days. Its BlackScholes price is...
-
Prescott Football Manufacturing had the following operating results for 2 0 1 9 : sales = $ 3 0 , 8 2 4 ; cost of goods sold = $ 2 1 , 9 7 4 ; depreciation expense = $ 3 , 6 0 3 ; interest expense =...
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...

Study smarter with the SolutionInn App


