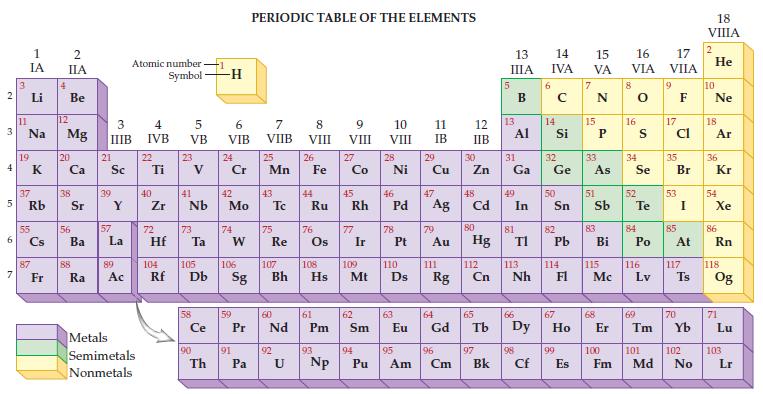
Given the only naturally occurring isotope of aluminum is Al-27, determine its mass from the periodic table.
Question:
Given the only naturally occurring isotope of aluminum is Al-27, determine its mass from the periodic table.
Transcribed Image Text:
2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55 87 Fr 4 2 IIA Be 12 Mg 20 38 56 Ba 88 Ra 3 IIIB 21 Sc 39 57 Y La 89 Ac Atomic number Symbol Metals Semimetals Nonmetals 4 IVB 22 Ti 40 Zr 5 VB 72 23 41 Nb 73 Hf Ta Ce 90 -1 Th -H 6 VIB 24 Cr 42 Mo 74 W 59 91 PERIODIC TABLE OF THE ELEMENTS 7 VIIB Pa 25 Mn 43 75 Re 60 P Pr Nd 92 8 VIII U 26 44 Fe Ru 76 61 Pm 93 9 VIII 104 105 106 107 108 109 110 Rf Db Sg Bh Hs Mt D Np 27 Co 45 Rh 77 Ir 62 Sm 94 10 VIII Pu 28 Ni 46 78 47 Pd Ag 63 11 IB 29 95 Cu 79 Au 111 64 Eu Gd Rg 96 Am Cm 12 IIB 30 Zn 48 Cd 80 Hg 112 Cn 65 97 Bk 13 IIIA 13 31 Ga Al 49 81 In 66 TI 113 Nh 98 Cf 6 14 15 16 IVA VA VIA 14 Si 32 Ge 50 82 Pb 114 67 99 E Es 15 33 As 51 83 Bi 115 Mc 68 8 16 34 Se 52 S Te 84 Po 116 Lv 69 17 VIIA 9 17 Cl 35 53 85 Br I At 117 70 Ts 100 101 102 Fm Md No 18 VIIIA 2 He 10 Ne 18 Ar 36 Kr 54 Xe 86 Rn 118 Og 71 Lu 103 Lr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Isotope Mass 26982amu A...View the full answer

Answered By

Muqadas Javed
I am a mentor by profession since seven years. I have been teaching on online forums and in universities. Teaching is my passion therefore i always try to find simple solution for complicated problems or task grasp them so that students can easily grasp them.I will provide you very detailed and self explanatory answers and that will help you to get good grade. I have two slogans: quality solution and on time delivery.
4.60+
24+ Reviews
144+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Given the only naturally occurring isotope of phosphorus is P-31, determine its mass from the periodic table. 2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55 87 Fr 4 2 IIA Be 12 Mg 20 38 56 Ba 88 Ra 3 IIIB...
-
Given the only naturally occurring isotope of fluorine is F-19, determine its mass from the periodic table. 2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55 87 Fr 4 2 IIA Be 12 Mg 20 38 56 Ba 88 Ra 3 IIIB 21...
-
Given the only naturally occurring isotope of sodium is Na-23, determine its mass from the periodic table. 2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55 87 Fr 4 2 IIA Be 12 Mg 20 38 56 Ba 88 Ra 3 IIIB 21...
-
In Exercises 130, find the domain of each function. f(x) = 1 4 x - 2 3
-
Magnificent Modems, Inc., makes modem cards that are used in notebook computers. The company completed the following transactions during 2010. All purchases and sales were made with cash. 1. Acquired...
-
Where do the DF originate for a SEM model?
-
Women in top management. The Journal of Organizational Culture, Communications and Conflict (July 2007) published a study on women in upper management positions at U.S. firms. Monthly data (n = 252...
-
Why is the statement of cash flows a useful document?
-
How does product market competitiveness affect risk exposure to multinational firms?
-
Calculate the atomic mass for lithium given the following data for its natural isotopes. 'Li: 7Li: 6.015 amu 7.016 amu 7.42% 92.58%
-
Draw a diagram of the arrangement of protons, neutrons, and electrons in an atom of each of the following isotopes. (a) 31 15 P (b) 35 17 Cl (c) 40 18 Ar (d) 131 5 3 I.
-
Regan Corporation reported the following amounts on its 2021 comparative income statements: Perform a horizontal analysis of revenues, expenses, and net incomeboth in dollar amounts and in...
-
Safeway, Inc., operated 1,739 stores as of January 3, 2009. The following data were taken from the company's annual report. All dollar amounts are in thousands. Required a. Compute Safeway's...
-
Rich French, the owner of Rich's Fishing Supplies, is surprised at the amount of actual inventory at the end of the year. He thought there should be more inventory on hand based on the amount of...
-
Carol Lapaz owned a small company that sold boating equipment. The equipment was expensive, and a perpetual system was maintained for control purposes. Even so, lost, damaged, and stolen merchandise...
-
The following footnote related to accounting for inventory was taken from the 2008 annual report of Wal-Mart, Inc. Inventories The Company values inventories at the lower of cost or market as...
-
Plot the magnitude and phase of the frequency response of normalized n-th order lowpass Butterworth filters.
-
Which of the following objects are chiral? a. A mug with DAD written on one side b. A mug with MOM written on one side c. A mug with DAD written opposite the handle d. A mug with MOM written opposite...
-
Which of the companies has the lowest accounts receivable turnover in the year 20X2? a. Company A. b. Company B. c. Company C. d. CompanyD. 20X1 20X2 Credit Sales Average Receivables Balance $1.0...
-
What is the net advantage to leasing (NAL)? Does your analysis indicate that Lewis should buy or lease the equipment? Explain. MINI CASE Lewis Securities Inc. has decided to acquire a new market data...
-
Now assume that the equipments residual value could be as low as $0 or as high as $400,000, but that $200,000 is the expected value. Since the residual value is riskier than the other cash flows in...
-
The lessee compares the cost of owning the equipment with the cost of leasing it. Now put yourself in the lessors shoes. In a few sentences, how should you analyze the decision to write or not write...
-
Which of the following programs covers custodial care? A HMOs B Medicare Part B C PPOs D Medicare Part A E Medicaid
-
uppose a taxpayer has exhausted his lifetime exclusion amount and has $14 million. a. Assuming a flat 40% gift tax rate, what is the maximum amount a taxpayer can transfer to her daughter (and still...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...

Study smarter with the SolutionInn App


