Given the only naturally occurring isotope of phosphorus is P-31, determine its mass from the periodic table.
Question:
Given the only naturally occurring isotope of phosphorus is P-31, determine its mass from the periodic table.
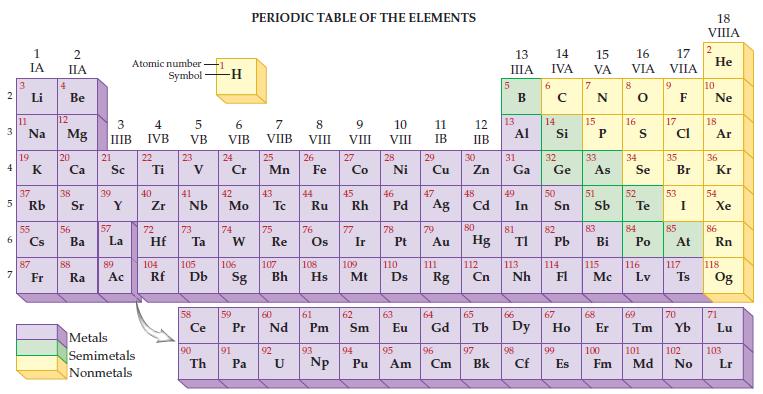
Transcribed Image Text:
2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55 87 Fr 4 2 IIA Be 12 Mg 20 38 56 Ba 88 Ra 3 IIIB 21 Sc 39 57 Y La 89 Ac Atomic number Symbol Metals Semimetals Nonmetals 4 IVB 22 Ti 40 Zr 5 VB 72 23 41 Nb 73 Hf Ta Ce 90 -1 Th -H 6 VIB 24 Cr 42 Mo 74 W 59 91 PERIODIC TABLE OF THE ELEMENTS 7 VIIB Pa 25 Mn 43 75 Re 60 P Pr Nd 92 8 VIII U 26 44 Fe Ru 76 61 Pm 93 9 VIII 104 105 106 107 108 109 110 Rf Db Sg Bh Hs Mt D Np 27 Co 45 Rh 77 Ir 62 Sm 94 10 VIII Pu 28 Ni 46 78 47 Pd Ag 63 11 IB 29 95 Cu 79 Au 111 64 Eu Gd Rg 96 Am Cm 12 IIB 30 Zn 48 Cd 80 Hg 112 Cn 65 97 Bk 13 IIIA 13 31 Ga Al 49 81 In 66 TI 113 Nh 98 Cf 6 14 15 16 IVA VA VIA 14 Si 32 Ge 50 82 Pb 114 67 99 E Es 15 33 As 51 83 Bi 115 Mc 68 8 16 34 Se 52 S Te 84 Po 116 Lv 69 17 VIIA 9 17 Cl 35 53 85 Br I At 117 70 Ts 100 101 102 Fm Md No 18 VIIIA 2 He 10 Ne 18 Ar 36 Kr 54 Xe 86 Rn 118 Og 71 Lu 103 Lr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The mass of an element listed on the periodic table ...View the full answer

Answered By

Emel Khan
I have the ability to effectively communicate and demonstrate concepts to students. Through my practical application of the subject required, I am able to provide real-world examples and clarify complex ideas. This helps students to better understand and retain the information, leading to improved performance and confidence in their abilities. Additionally, my hands-on approach allows for interactive lessons and personalized instruction, catering to the individual needs and learning styles of each student.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Given the only naturally occurring isotope of fluorine is F-19, determine its mass from the periodic table. 2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55 87 Fr 4 2 IIA Be 12 Mg 20 38 56 Ba 88 Ra 3 IIIB 21...
-
Given the only naturally occurring isotope of aluminum is Al-27, determine its mass from the periodic table. 2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55 87 Fr 4 2 IIA Be 12 Mg 20 38 56 Ba 88 Ra 3 IIIB 21...
-
Given the only naturally occurring isotope of sodium is Na-23, determine its mass from the periodic table. 2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55 87 Fr 4 2 IIA Be 12 Mg 20 38 56 Ba 88 Ra 3 IIIB 21...
-
In Exercises 1126, determine whether each equation defines y as a function of x. x + y = 16
-
How can knowing cost behavior relative to volume fluctuations affect decision making?
-
What is MANOVA? What distinguishes MANCOVA from MANOVA?
-
Violent behavior in children. Refer to the Development Psychology (Mar. 2003) study of the behavior of elementary school children, presented in Exercise 8.149 (p. 454). The researchers used a...
-
For each of the following costs, check the columns that most likely apply (both variable and fixed might apply for somecosts). Product Costs Direct nect Period Variable Fixed Raw materials Staples...
-
Current Position Analysis PepsiCo, Inc. (PEP), the parent company of Frito Lay snack foods and Pepsi beverages, had the following current assets and current liabilities at the end of two recent...
-
Calculate the atomic mass for lithium given the following data for its natural isotopes. 'Li: 7Li: 6.015 amu 7.016 amu 7.42% 92.58%
-
Draw a diagram of the arrangement of protons, neutrons, and electrons in an atom of each of the following isotopes. (a) 31 15 P (b) 35 17 Cl (c) 40 18 Ar (d) 131 5 3 I.
-
In 2010, Cinnamons net profit margin would be highest if: A. it is deemed to have control of Cambridge. B. it had not increased its stake in Cambridge. C. it is deemed to have significant influence...
-
Prove (11.32) . E (Yi,k | Zi = 0, = e) = E (Yi,k | i = 1, = e) = E (Yi,k | Ti = e), k = 1,2. (11.32)
-
University Medical Center needs to move from its existing facility to a new and larger facility five miles away from its current location. Due to construction delays, however, much of the new...
-
Calculate the base value or lump sum for each of the single and married filing jointly 2016 brackets given in Table 6.4. Table 6.4 ITABLE 6.4 Corporate Income Brackets and Tax Rates, 2015 Taxable...
-
Show that staged column diameter is proportional to (feed rate) \({ }^{1 / 2}\) and to \((1+\mathrm{L} / \mathrm{D})^{1 / 2}\).
-
An atmospheric column with 25 real stages is operating with a pressure drop of 0.6 in. of water per stage. Assume pressure drop in the condenser and the reboiler is \(1.2 \mathrm{in}\). of water...
-
Give the products of the following reactions. If the products can exist as stereoisomers, show which stereoisomers are obtained. a. Cis-2-pentene + Br2JCH2CI2 b. Trans.2-pcn(cnc + Br2CH2C1 c....
-
What types of questions can be answered by analyzing financial statements?
-
How are leases classified for tax purposes? MINI CASE Lewis Securities Inc. has decided to acquire a new market data and quotation system for its Richmond home office. The system receives current...
-
What effect does leasing have on a firms balance sheet? MINI CASE Lewis Securities Inc. has decided to acquire a new market data and quotation system for its Richmond home office. The system receives...
-
What effect does leasing have on a firms capital structure? MINI CASE Lewis Securities Inc. has decided to acquire a new market data and quotation system for its Richmond home office. The system...
-
Hite corporation intends to issue $160,000 of 5% convertible bonds with a conversion price of $40 per share. The company has 40,000 shares of common stock outstanding and expects to earn $600,000...
-
Your portfolio has a beta of 1.17, a standard deviation of 14.3 percent, and an expected return of 12.5 percent. The market return is 11.3 percent and the risk-free rate is 3.1 percent. What is the...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...

Study smarter with the SolutionInn App


