Predict the missing value (?) for each physical property listed below. The (a) Atomic radius, (b) Density,
Question:
Predict the missing value (?) for each physical property listed below. The
(a) Atomic radius,
(b) Density,
(c) Melting point are given for two of three alkaline earth metals in Group IIA/2.
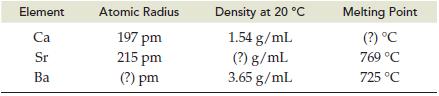
Transcribed Image Text:
Element Ca Sr Ba Atomic Radius 197 pm 215 pm (?) pm Density at 20 °C 1.54 g/mL (?) g/mL 3.65 g/mL Melting Point (?) °C 769 °C 725 °C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
We can estimate a value for the physical property of an element by observing the trend in values for ...View the full answer

Answered By

Simranjeet kaur Sangatni
Accountancy is my favorite subject. I worked as Asst. Accountant in Sanjay Pandya Accountancy Firm and also as Faculty of Accountancy in Reliable Eduworld for 11th and 12th CBSE and for CA CPT entrance. I used to take private classes also for 12th and B.com students. I also worked as freelancing in solution providing company.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
What are the expected readings of the ammeter and voltmeter for the circuit inFigure? 6.00 2 10.0 2 6.00 V 5.00 2 4.50 V 6.00 2
-
Predict the missing value (?) for each physical property listed below. The (a) Atomic radius, (b) Density, (c) Melting point are given for two of the metals in Group VIII/10. Element Ni Pd Pt Atomic...
-
Predict the missing value (?) for each property listed below. The atomic radius, density, and melting point are given for elements in Group VIII/8. Element Fe Ru Os Atomic Radius 126 pm (?) pm 135 pm...
-
When Ralph Lauren makes shirts to a customers exact preferences, what utility is provided?
-
What is an annuity agreement and how does it differ from a life income fund?
-
The siphon of Problem 6.31 is made of smooth rubber hose and is 23 ft long. Determine the flow rate and the pressure at point B.
-
8-5. What factor is estimated or measured for each of the cells in a marketproduct grid?
-
Morray Corporation had the following transactions. 1. Issued $160,000 of bonds payable. 2. Paid utilities expense. 3. Issued 500 shares of preferred stock for $45,000. 4. Sold land and a building for...
-
please show work 8. Clark company reported the following balances at June 30, the end of the fiscal year. Net sales>20,200 cost of goods sold>5,000 operating expenses>2,500 inverse revenue> 700...
-
Why did Mendeleev not include einsteinium in his periodic table of 1871? Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr...
-
Given the density of silver (10.5 g/cm 3 ) and gold (19.3 g/cm 3 ) in Group IB/11, estimate the density for the synthetic element roentgenium, which is below gold in the periodic table. Periodic...
-
A solid-state camera has a 256 x 256 pixel matrix. The analogtodigital converter takes 0.20 microseconds (0.20 x 10-6 sec) to convert the analog charge signal for each pixel into the corresponding...
-
Part 1 of 4 05 points abook Print References Required information Problem 24-2A (Algo) Payback period, accounting rate of return, net present value, and net cash flow calculation LO P1, P2, P3 [The...
-
Keenan Music's CEO has been pondering about the recent proposal of the Specialty Guitar Project. The accountant has done a capital budgeting analysis on the project and outlined the conditions that...
-
On July 1, 2025, Sheridan Co. pays $15,000 to Blue Spruce Insurance Co. for a 2-year insurance contract. Both companies have fiscal years ending December 31. (a1) Journalize the entry on July 1 and...
-
A CU triaxial test with c = 20 psi is performed on a sand and a deviator stress of 80 psi fails the specimen. Previous tests revealed that the effective friction angle for this sand is 35. Calculate...
-
Haliburton Mills Inc. is a large producer of men's and women's clothing. The company uses standard costs for all of its products. The standard costs and actual costs for a recent period are given...
-
Wolvo Company has defective products in inventory. It has the opportunity to either sell, scrap, or rebuild the defective products. Identify several factors Wolvo Company should consider before...
-
Suppose that the electrical potential at the point (x, y, z) is E(x, y, z) = x + y - 2z. What is the direction of the acceleration at the point (1,3,2)?
-
Teresa Ramirez and Lenny Traylor are examining the following statement of cash flows for Pacific Clothing Stores first year of operations. Teresa claims that Pacifics statement of cash flows is an...
-
Ashley Company is a young and growing producer of electronic measuring instruments and technical equipment. You have been retained by Ashley to advise it in the preparation of a statement of cash...
-
Each of the following items must be considered in preparing a statement of cash flows for Cruz Fashions Inc. for the year ended December 31, 2010. 1. Fixed assets that had cost $20,000 612 years...
-
If John invested $20,000 in a stock paying annual qualifying dividends equal to 4% of his investment, what would the value of his investment be 5 years from now? Assume Johns marginal ordinary tax...
-
help asap please!
-
Please, help asap! I have one day. Feedback will be given. & show some work. [in Excel] For the final project you will need you to create a spreadsheet /proforma of the cash flows from a property....

Study smarter with the SolutionInn App


