Predict the missing value (?) for radioactive astatine (At). The atomic radius, density, and boiling point are
Question:
Predict the missing value (?) for radioactive astatine (At). The atomic radius, density, and boiling point are given for elements in Group VIIIA/17.
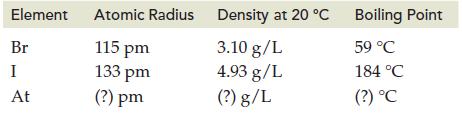
Transcribed Image Text:
Element Br I At Atomic Radius 115 pm 133 pm (?) pm Density at 20 °C Density at 20 °C 3.10 g/L 4.93 g/L (?) g/L Boiling Point 59 °C 184 °C (?) °C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The missing values for Astatine At can be predicted based on the trends observed in Group VIIA17 ele...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Predict the missing value (?) for radioactive radon (Rn). The atomic radius, density, and boiling point are given for elements in Group VIIIA/18. Element Kr Xe Rn Atomic Radius 202 pm 210 pm (?) pm...
-
Predict the missing value (?) for each property listed below. The atomic radius, density, and boiling point are given for elements in Group VIB/6. Element Cr Mo W Atomic Radius 125 pm (?) pm 137 pm...
-
Predict the missing value (?) for radioactive radium (Ra). The atomic radius, density, and melting point are given for elements in Group IIA/2. Element Atomic Radius 215 pm 217 pm (?) pm Sr Ba Ra...
-
Give the decoding key for the encoding keys in Problems 2536. Minus 4, divide by 2.
-
Explain why conventional cost accounting systems have become less useful in both business and government settings. How does activity-based costing (ABC) reduce the problems created by conventional...
-
When a string barely strong enough lifts a heavy weight, it can lift the weight by a steady pull; but if you jerk the string, it will break. Explain in terms of Newtons laws of motion.
-
The text describes three characteristics of demand. Name and describe each. LO.1
-
On January 1, 2010, Pine Grove Country Club purchased a new riding mower for $15,000.The mower is expected to have an 8-year life with a $1,000 salvage value . What journal entry would Pine Grove...
-
Perfect Pet Collar Company makes custom leather pet collars. The company expects each collar to require 200 feet of leather and predicts leather will cost $3.50 per foot Suppose Perfect Pet made 50...
-
The chemical formula for barium chloride is BaCl 2 . Predict the formulas for each of the following similar compounds. (a) Calcium fluoride (b) Calcium chloride (c) Calcium bromide (d) Calcium iodide.
-
The formulas for the oxides of sodium, magnesium, aluminum, and silicon are, respectively, Na 2 O, MgO, Al 2 O 3 , and SiO 2 . Using the periodic table, predict the chemical formulas for each of the...
-
When it is heated, potassium chlorate decomposes to potassium chloride and oxygen: A sample of solid KClO 3 decomposes to produce 150.0 mL of oxygen at 22.0C and 780.5 mm Hg. How many grams of KClO 3...
-
The residents of the town of Stewart are well-known coffee drinkers. Dunkin Donuts and Starbucks stores supply the residents with over 100,000 cups of hot coffee per week. Recently, coffee drinkers...
-
Read through the following case study and analyse it by addressing the question that follows. Case study: Adriana Adriana runs a very busy multi-sports coaching company that provides after-school...
-
Assuming that you were invited to one of your best friends' wedding. Therefore, you decided to purchase a special product or service as a gift for this important event. Based on this scenario, you...
-
The Department of Commerce, through the Economic Development Administration (EDA), is seeking information to inform the planning and design of the Regional Technology and Innovation Hub (Tech Hubs)...
-
In frontline management, it is essential to manage time effectively. In the table below, list 3 time-management principles that are relevant to a frontline manager's own work and provide 3 examples...
-
Shelby has net investment income of $18,450 and wage income of $76,000. She paid investment interest expense of $19,000. What is Shelby's deduction for investment interest expense? Explain your...
-
We all experience emotions, but some people disguise their true feelings better than others. Do you think this is a helpful or harmful thing to do? Under what conditions do you think it would be most...
-
(Permanent and Temporary Differences, One Rate) The accounting records of Shinault Inc. show the following data for 2010. 1. Life insurance expense on officers was $9,000. 2. Equipment was acquired...
-
(NOL without Valuation Account) Jennings Inc. reported the following pretax income (loss) and related tax rates during the years 2006' ?2012. Pretax financial income (loss) and taxable income (loss)...
-
Two Differences, Two Rates, Future Income Expected) Presented below are two independent situations related to future taxable and deductible amounts resulting from temporary differences existing at...
-
The following is part of the computer output from a regression of monthly returns on Waterworks stock against the S&P 5 0 0 index. A hedge fund manager believes that Waterworks is underpriced, with...
-
Doisneau 25-year bonds have an annual coupon interest of 8 percent, make interest payments on a semiannual basis, and have a $1,000 par value. If the bonds are trading with a market's required yield...
-
Hite corporation intends to issue $160,000 of 5% convertible bonds with a conversion price of $40 per share. The company has 40,000 shares of common stock outstanding and expects to earn $600,000...

Study smarter with the SolutionInn App


