The formulas for the oxides of sodium, magnesium, aluminum, and silicon are, respectively, Na 2 O, MgO,
Question:
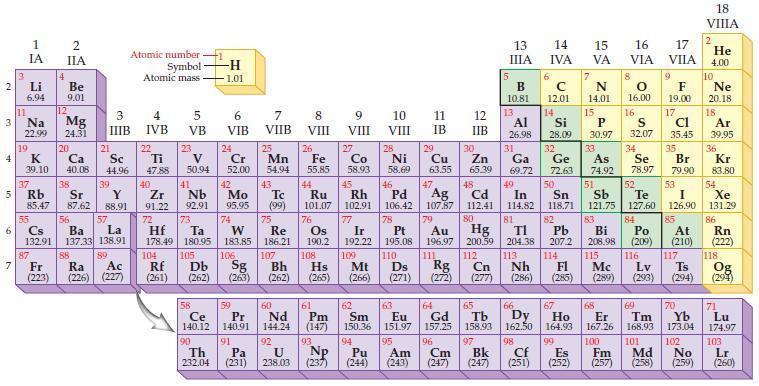
The formulas for the oxides of sodium, magnesium, aluminum, and silicon are, respectively, Na2O, MgO, Al2O3, and SiO2. Using the periodic table, predict the chemical formulas for each of the following similar compounds.
(a) Lithium oxide
(b) Barium oxide
(c) Gallium oxide
(d) Tin oxide.
Periodic Table:
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 117 116 Mc Ts Lv (289) (293) (294) 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
Answers The chemical formulas for oxides are determined by the combining ratios of the elements invo...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Arthur Korrey is developing a project to start a new professional aquatic baseball league, with teams comprised of humans, dolphins and penguins. He forecasts that net annual cash flows will be zero...
-
Calculate the CCl and CC bond lengths in ethyl chloride, C 2 H 5 Cl, using values for the covalent radii from Table 9.4. How do these values compare with the experimental values: CCl, 177; CC, 155...
-
What do you expect for the BCl bond length in boron trichloride, BCl 3 , on the basis of covalent radii (Table 9.4)? Table 9.4 Single-Bond Covalent Radii Atomic Covalent Atomic Covalent Number Symbol...
-
Use the trapezium rule, with interval-halving and extrapolation, to evaluate 0 log(cosh x) dx to 4dp
-
What are the three broad categories of service efforts and accomplishments (SEA) measures? Explain the GASBs role in developing standards for SEA reporting.
-
Why is it incorrect to say that 1.0 kg equals 2.2 lb?
-
Describe trend, seasonality, random variation, and cycle as applied to forecasting. LO.1
-
1. Using the following information, compute the cost of direct materials used. Raw materials inventory, January 1 ........ $30,000 Raw materials inventory, December 31 ...... $60,000 Work in process,...
-
LIFO conformity rule state that if LIFO is used for
-
Predict the missing value (?) for radioactive astatine (At). The atomic radius, density, and boiling point are given for elements in Group VIIIA/17. Element Br I At Atomic Radius 115 pm 133 pm (?) pm...
-
Predict the missing value (?) for radioactive radium (Ra). The atomic radius, density, and melting point are given for elements in Group IIA/2. Element Atomic Radius 215 pm 217 pm (?) pm Sr Ba Ra...
-
One powerful feature of constraint programming is that variables can be used as subscripts for the terms in the objective function. For example, consider the following traveling salesman problem. The...
-
How do emergent properties within teams, such as synergy and collective intelligence, manifest and influence team performance, and what factors contribute to their development and sustenance?
-
Think about your workplace, organization, or industry; if you are not currently working, think about previous employment or a job that you are aiming for. The broader your perspective, the more...
-
Choose any global organization that successfully undertook a strategic transformation to adapt to changing market dynamics and sustain its competitive advantage. Examine the company's challenges,...
-
In examining C&C Sports through the lens of a SWOT analysis, several key factors come to light. The strengths of the company are evident in its established brand reputation and a loyal customer base...
-
A perfectly insulated container initially contains 0.2 kg of ice at -15 C. Now we add water at 30 C, but only the minimum amount needed to barely melt all the ice. Find the net entropy change of the...
-
What are the six types of personal expenses that can be classified as itemized deductions on Schedule A, Form 1040?
-
CdF2 (s) Cd+ (aq) + 2 F- (aq) 1. A saturated solution of CdF2 is prepared. The equilibrium in the solution is represented above. In the solution [Cd+] eq = 0.0585 M and [F-] eq = 0.117 M. a....
-
(One Temporary Difference, Tracked 3 Years, Change in Rates, Income Statement Presentation) Crosley Corp. sold an investment on an installment basis. The total gain of $60,000 was reported for...
-
Two Differences, 2 Years, Compute Taxable Income and Pretax Financial Income) The following information was disclosed during the audit of Elbert Inc. 2. On January 1, 2010, equipment costing $600,000...
-
(Five Differences, Compute Taxable Income and Deferred Taxes, Draft Income Statement) Wise Company began operations at the beginning of 2011. The following information pertains to this company. 1....
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...
-
Wendell's Donut Shoppe is investigating the purchase of a new $39,600 conut-making machine. The new machine would permit the company to reduce the amount of part-time help needed, at a cost savings...
-
1.Discuss the challenges faced with Valuing Stocks and Bonds. As part of this discussion, how will the selected item be implemented in an organization and its significance? 2. Discuss how Valuing...

Study smarter with the SolutionInn App


