Predict which element in each of the following pairs is more electronegative according to the general trends
Question:
Predict which element in each of the following pairs is more electronegative according to the general trends in the periodic table.
(a) Se or Br
(b) C or B
(c) Te or S
(d) Ba or Be.
Periodic Table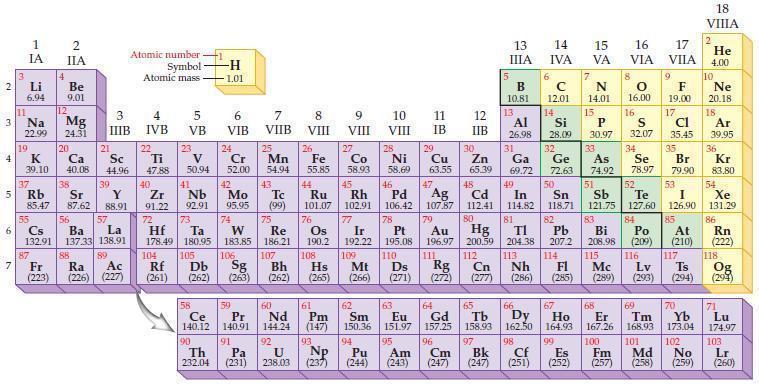
Transcribed Image Text:
2 3 4 5₁ 6 7 3 11 Li 6.94 1 IA Na 22.99 19 37 al 55 4 87 2 IIA Fr (223) Be 9.01 12 K Ca Sc 39.10 40.08 44.96 Mg 24.31 38 Rb Sr Y 85.47 87.62 88.91 56 57 Cs Ba La 132.91 137.33 138.91 20 21 88 3 IIIB 39 89 Atomic number Symbol - Ac Ra (226) (227) Atomic mass 4 IVB 22 Ti 47.88 40 5 VB 23 V 50.94 41 Zr Nb 91.22 92.91 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 72 73 W Re Hf Ta 178.49 180.95 183.85 186.21 91 7 VIIB Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 108 9 VIII 61 Pm (147) 27 Bh Hs Mt (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB Pt 195.08 110 29 Cu 63.55 13 IIIA 12 IIB 5 B 10.81 13 17 16 VA VIA VIIA 8 Al 26.98 6 C 12.01 14 Si 28.09 32 30 31 33 As Se Br Zn Ga Ge 65.39 69.72 72.63 74.92 78.97 79.90 14 15 IVA 7 N 14.01 15 P 30.97 16.00 83 Bi 208.98 115 16 S 32.07 34 84 47 48 49 50 51 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 79 Au Hg 196.97 200.59 111 112 Rg Cn (271) (272) (277) (286) (285) (289) (293) (294) 81 82 TI Pb 204.38 207.2 113 114 Nh Fl Ds Mc Lv Ts Po (209) 9 116 F 19.00 Md (258) 17 Cl 35.45 35 53 I 126.90 85 At (210) 66 67 69 70 63 64 65 68 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 98 99 100 Cf Es Fm (251) (252) (257) 117 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Electronegativity Predictions based on Periodic Trends Heres my prediction for each pair based on ge...View the full answer

Answered By

Simon kingori
I am a tier-one market researcher and content developer who has been in this field for the last six years. I’ve run the freelancing gamut; from market research, data mining and SEO/SMM to copywriting, Content Development, you name it, I’ve done it. I’m extremely motivated, organized and disciplined – you have to be to work from home. My experience in Freelancing is invaluable- but what makes me a cut above the rest is my passion to deliver quality results to all my clients- it’s important to note, I've never had a dissatisfied client. Backed by a Masters degree in Computer Science from MOI university, I have the required skill set and burning passion and desire to deliver the best results for my clients. This is the reason why I am a cut above the rest. Having taken a Bsc. in computer science and statistics, I deal with all round fields in the IT category. It is a field i enjoy working in as it is dynamic and new things present themselves every day for research and exploration.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Predict which element in each of the following pairs is more electronegative according to the general trends in the periodic table. (a) Br or Cl (b) O or S (c) Se or As (d) N or F. Periodic Table 2 3...
-
Predict which element in each of the following pairs is more electronegative according to the general trends in the periodic table: (a) H or Cl (b) Br or I (c) P or S (d) As or Sb.
-
Predict which element in each of the following pairs is more electronegative according to the general electronegativity trends in the periodic table: (a) N or O (b) Br or Se (c) F or Cl (d) Si or C.
-
Governmental Funds Statement of Revenues Expenditures and Changes in Fund Balance. You have recently started working as the controller for a small county. The county is preparing its financial...
-
List the authoritative documents available to financial statement preparers and auditors related to governmental accounting and financial reporting. Which is the most authoritative according to GASB...
-
Refer to Exercise 61. Calculate the amount of the noncontrolling interest to be deducted from consolidated income in arriving at 2019 controlling interest in consolidated net income. Exercise 61 P...
-
According to researchers, diabetes is rare in societies in which obesity is rare. In societies in which obesity has been common for at least 20 years, diabetes is also common. (Source: American...
-
The chocolate crumb mystery explosions ignited by electrostatic discharges (sparks) constitute a serious danger in facilities handling grain or powder. Such an explosion occurred in chocolate crumb...
-
Middleton Associates is a consulting firm that specializes in information systems for construction and landscaping companies. The firm has two officesone in Toronto and one in Vancouver. The firm...
-
Which elements are more electronegative: semimetals or nonmetals?
-
Write the electron dot formula and draw the structural formula for each of the following polyatomic ions. (a) PH 4 + (b) SeO 3 2 (c) CO 3 2 (d) BO 3 3
-
Give examples of areas where conflicts of interest may occur.
-
1) Factor the following Expressions (Write your factors only, don't show your work) a) 2x - 32 = c) 3x-2x-8= b) 2x-6x-8=
-
Bloomfield Inc. manufactures widgets. A major piece of equipment used to make the widget is nearing the end of its useful life. The company is trying to decide whether they should lease new equipment...
-
1. a. What is network management? Illustrate network management functional flowchart. [2.5] b. What encoding and decoding mechanisms are used in fast Ethernet and gigabit Ethernet? What is meant by...
-
Project Data: Sam Parker owns and operates a consulting firm called Business Solutions. The business began operating in October 202X. Transactions for October and November 202X have been recorded and...
-
3. Use Hooke's law to predict which one out of each pair vibrates at a higher wavenumber. Explain your answer. (7 points) a) C-H and C-D* b) C-C and C=C where: 1 k v = 2, v=wavenumber c = velocity of...
-
A homebuilder's advertising has the caption, "Inflation to Continue for Many Years." The advertisement continues with the explanation that if one buys a home now for $97,000, and inflation continues...
-
The column shown in the figure is fixed at the base and free at the upper end. A compressive load P acts at the top of the column with an eccentricity e from the axis of the column. Beginning with...
-
Calculating net float each business day, on average, a company writes checks totaling $17,000 to pay its suppliers. The usual clearing time for the checks is four days. Meanwhile, the company is...
-
Costs of Float Purple feet wine, Inc., receives an average of $11,000 in checks per day. The delay in clearing is typically four days. The current interest rate is .016 percent per day. a. What is...
-
Float and Weighted Average Delay your neighbor goes to the post office once a month and picks up two checks, one for $13,000 and one for $4,000. The larger check takes four days to clear after it is...
-
ABC Corporation has an activity - based costing system with three activity cost pools - Machining, Setting Up , and Other. The company's overhead costs, which consist of equipment depreciation and...
-
Consolidated Balance Sheets - USD ( $ ) $ in Thousands Dec. 3 1 , 2 0 2 3 Dec. 3 1 , 2 0 2 2 Current assets: Cash and cash equivalents $ 9 8 , 5 0 0 $ 6 3 , 7 6 9 Restricted cash 2 , 5 3 2 Short -...
-
How does corporate governance contribute to investor confidence and stakeholder trust? Accounting

Study smarter with the SolutionInn App


