Refer to a periodic table and write the predicted electron configuration for each of the following elements.
Question:
Refer to a periodic table and write the predicted electron configuration for each of the following elements.
(a) Zn
(b) Se.
Periodic Table:
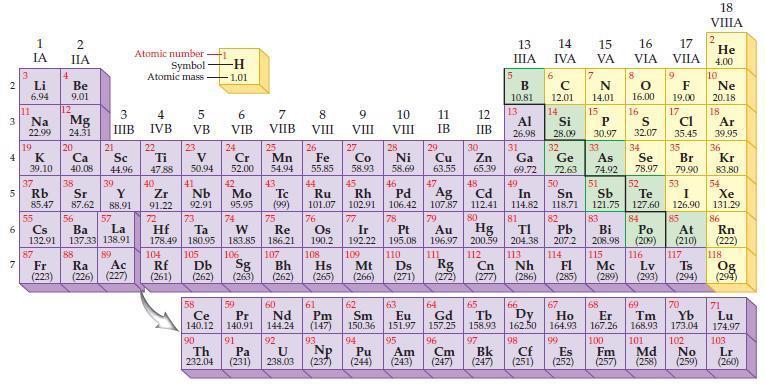
Transcribed Image Text:
2 3 4 15 6 7 3 11 Li 6.94 1 IA Na 22.99 19 37 R Rb 4 87 2 IIA Be 9.01 12 Mg 24.31 K Ca Sc 39.10 40.08 44.96 20 38 21 3 IIIB 39 Sr Y 85.47 87.62 88.91 57 55 56 La Cs Ba 132.91 137.33 138.91 89 88 Fr Ac Ra (223) (226) (227) Atomic number Symbol Atomic mass 4 IVB 22 Ti 47.88 40 Zr 2 91.22 72 5 VB 104 23 V 50.94 41 73 Hf Ta 178.49 180.95 105 Rf Db (261) (262) -H -1.01 6 VIB Nb Mo 92.91 95.95 90 24 Cr 52.00 42 74 106 Sg (263) 58 Pr Ce 140.12 140.91 91 59 7 VIIB Th Pa 232.04 (231) 25 W Re 183.85 186.21 Mn 54.94 43. Tc (99) 75 107 Bh (262) 60 Nd 144.24 92 U 238.03 8 VIII 26 Fe 55.85 76 Os 190.2 61 Pm (147) 93 Np 9 VIII (237) 10 VIII 11 IB 12 IIB 27 28 29 Zn Co Ni Cu 58.93 58.69 63.55 65.39 44 49 45 46 48 Ru Rh Pd Cd In 101.07 102.91 106.42 107.87 112.41 114.82 Ag 77 78 79 80 Ir Pt 192.22 195.08 109 110 Hs Mt Ds Rg Cn (265) (266) (271) (272) (277) Au Hg 196.97 200.59 111 112 108 47 30 5 13 ΠΙΑ B 10.81 13 6 14 IVA с 12.01 14 15 16 17 VA VIA VILA 7 N 14.01 15 66 67 62 63 64 65 Sm Eu Gd Tb Dy Ho 150.36 151.97 157.25 158.93 162.50 164.93 99 94 95 96 97 Pu Am Cm Bk (244) (243) (247) (247) 8 O 16.00 16 Al Si P 30.97 31 34 26.98 28.09 32 33 Ga Ge As Se 69.72 72.63 74.92 78.97 50 51 52 53 Sn Sb Te I 118.71 121.75 127.60 126.90 82 83 84 85 TI Pb Bi Po At 204.38 207.2 208.98 (209) (210) 113 114 115 116 117 Nh Fl Lv Ts (286) (285) (289) (293) (294) 81 Mc 9 F 19.00 17 CI 35.45 S 32.07 35 Br 79.90 68 69 70 Er Tm Yb 167.26 168.93 173.04 100 101 102 Cf Es Fm Md No (251) (252) (257) 98 (258) (259) 18 VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39,95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 Lr (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
a 1s 2 2s 2 2p 6 3s 2 3...View the full answer

Answered By

Wahome Michael
I am a CPA finalist and a graduate in Bachelor of commerce. I am a full time writer with 4 years experience in academic writing (essays, Thesis, dissertation and research). I am also a full time writer which assures you of my quality, deep knowledge of your task requirement and timeliness. Assign me your task and you shall have the best.
Thanks in advance
4.90+
63+ Reviews
132+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to a periodic table and write the predicted electron configuration for each of the following elements by counting the number of electrons in each block: (a) P (b) Co. Periodic Table: 2 3 4 5 6...
-
Refer to the periodic table and write the predicted electron configuration for each of the following elements using core notation. (a) W (b) Bi (c) Ra (d) Ac. Periodic Table: 2 3 4 10 6 3 7 11 Li...
-
Refer to the periodic table and write the predicted electron configuration for each of the following elements using core notation. (a) Sr (b) Ru (c) Sb (d) Cs. Periodic Table: 2 3 4 10 6 3 7 11 Li...
-
What are the concepts of traditional and contemporary organizational design? Will these designs be influenced differently by management and the environment?
-
What are the required financial statements for? (a) A not-for-profit health care entity and (b) A governmental health care entity reporting only business-type activities?
-
A cross-flow molecular filtration device equipped with a mesoporous membrane is used to separate the enzyme lysozyme from a fermentation broth, as shown in the figure (right column). Water at...
-
What might make a project unsuccessful during the termination process? LO4
-
Kirby Railroad Co. is about to issue $300,000 of 10-year bonds paying a 9% interest rate, with interest payable semiannually. The discount rate for such securities is 8%. How much can Kirby expect to...
-
The same question posted. Has the wrong answer. So please make sure you have the correct answer Pharoah Corporation acquired new equipment at a cost of $109,000 plus 7% provincial sales tax and 5%...
-
Refer to the periodic table and predict the number of 5d electrons in a Pt atom. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 87 4 Fr (223) 2 IIA Be 9.01 12 Mg...
-
State the energy sublevel being filled in each of the following series of elements: (a) Cs - Ba (b) Y - Cd (c) In - Xe (d) Ce - Lu.
-
The T accounts for Equipment; Accumulated Depreciation, Equipment; and Loss on Disposal of Property and Equipment for Zhang Company at the end of 2013 are shown below. New equipment was bought for...
-
4. Write short notes on Wiener Filtering.
-
1.Explain Histogram processing
-
2. Explain Spatial Filtering ?
-
3. Explain the Geometric Transformations used in image restoration. 4.Describe homomorphic filtering
-
5.Explain the different Noise Distribution in detail. UNIT I V 1. What is segmentation? 2. Write the applications of segmentation. 3. What are the three types of discontinuity in digital image? 4....
-
Harry Blackmun, manager of the Dry Goods Department at Goodright's Grocery, has a budget of $6,000 per month for the current year. This budget includes the allocation of $500 of storewide common...
-
A sprinkler head malfunctions at midfield in an NFL football field. The puddle of water forms a circular pattern around the sprinkler head with a radius in yards that grows as a function of time, in...
-
What are some of the key obstacles for the FASB and IASB in their convergence project for the statement of cash flows?
-
Wainwright Corporation had the following activities in 2010. 1. Sale of land.............................................................$180,000 2. Purchase of...
-
Stansfield Corporation had the following activities in 2010. 1. Payment of accounts payable.............................$770,000 2. Issuance of common stock..................................$250,000...
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


