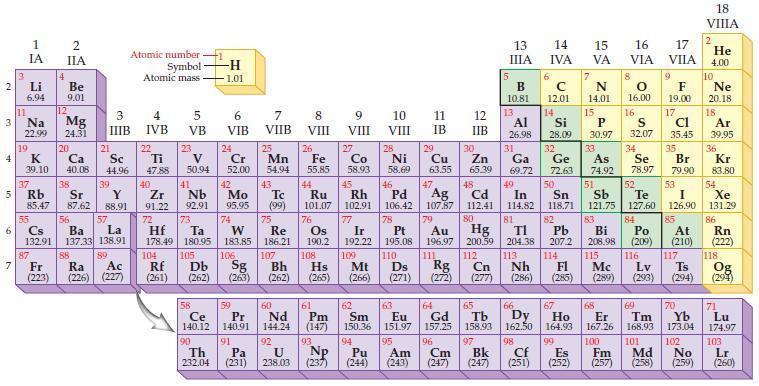
Refer to the periodic table and write the predicted electron configuration for each of the following elements
Question:
Refer to the periodic table and write the predicted electron configuration for each of the following elements using core notation.
(a) Sr
(b) Ru
(c) Sb
(d) Cs.
Periodic Table:
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 117 116 Mc Ts Lv (289) (293) (294) 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
Here are the predicted electron configurations for each of the elements in your question using core ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and write the predicted electron configuration for each of the following elements. (a) B (b) Ti (c) Na (d) O (e) Ge (f) Ba (g) Pd (h) Kr. Periodic Table: 2 3 4 10 6 3 7 11...
-
Refer to the periodic table and write the predicted electron configuration for each of the following elements. (a) Li (b) F (c) Mg (d) P (e) Ca (f) Mn (g) Ga (h) Rb. Periodic Table: 2 3 4 10 6 3 7 11...
-
Refer to the periodic table and write the predicted electron configuration for each of the following elements using core notation. (a) W (b) Bi (c) Ra (d) Ac. Periodic Table: 2 3 4 10 6 3 7 11 Li...
-
From the densities of the lines in the mass spectrum of krypton gas, the following observations were made: Somewhat more than 50% of the atoms were krypton-84. The numbers of krypton-82 and...
-
Accounting and Reporting Principles. For more than 100 years, the financial statements of the Town of Brookfield have consisted of a statement of cash receipts and a statement of cash disbursements...
-
An old mining tunnel disappears into a hillside. You would like to know how long the tunnel is, but its too dangerous to go inside. Recalling your recent physics class, you decide to try setting up...
-
Writing In a game of chance, what is the relationship between a "fair bet" and its expected value? Explain. Finding Expected Value In Exercises 35-40, use the probability distribution or histogram to...
-
Steve, Carol, and Lisa get their first full-time jobs and talk about saving for retirement. They are each 22 years old and plan to work until they are 55. Steve starts investing immediately and puts...
-
The City of Jonesboro engaged in the following transactions during the fiscal year ended September 30, 2018. REQUIRED: Record the following transactions related to interfund transfers. Be sure to...
-
Supply a systematic name for each of the following monoatomic anions: (a) Br (b) N 3 .
-
Predict the missing value (?) for each property listed below. The atomic radius, density, and melting point are given for elements in Group VIII/8. Element Fe Ru Os Atomic Radius 126 pm (?) pm 135 pm...
-
A converging-diverging nozzle with a 4:1 exit-area ratio and p0 = 500 kPa operates in an under expanded condition (case 1 of Fig. 9.12) as in Fig. P9.147. The receiver pressure is pa = 10 kPa, which...
-
What is current divider rule?Explain with a suitable example.
-
How do we design a Successive Approximation Register using PSPICE software?
-
1.Differentiate between a leader and a manager. 2.Highlight the sources of leadership power. 3.Highlight the styles of leadership and the one which is applicable in a small business. 4.Examine some...
-
Potassium has an atomic number of 19 and one unpaired electron. What orbital does the unpaired electron occupy?
-
CLASS PERIO Solving Linear Equations: Variable on Both Sides Solve each equation. 1) 6r+ 7 = 13 + 7r 3) -7x-3x+2=-8x-8 5)-14 +66+7-26=1+5b 7) n-3n = 14-4n 2) 13-4x=1-x 4)-8-x= x - 4x 6)n+2=-14-n 8)...
-
For each period that an income statement is presented, what must a company disclose about its EPS?
-
If a companys outstanding shares are increased through a stock dividend or a stock split, how would that alter the presentation of its EPS data?
-
(Warrants Issued with Bonds and Convertible Bonds) Incurring long-term debt with an arrangement whereby lenders receive an option to buy common stock during all or a portion of the time the debt is...
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


