Refer to the periodic table and write the predicted electron configuration for each of the following elements
Question:
Refer to the periodic table and write the predicted electron configuration for each of the following elements using core notation.
(a) W
(b) Bi
(c) Ra
(d) Ac.
Periodic Table:
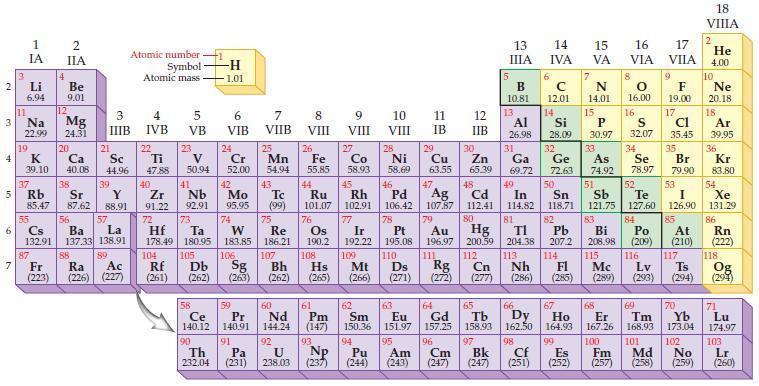
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 117 116 Mc Ts Lv (289) (293) (294) 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
Explanation a W74 ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and write the predicted electron configuration for each of the following ions using core notation: (a) Fe 3+ (b) Se 2 . 2 3 4 5 6 7 Li 6.94 11 1 IA Na 22.99 19 37 55 4 2...
-
Refer to the periodic table and write the predicted electron configuration for each of the following elements. (a) B (b) Ti (c) Na (d) O (e) Ge (f) Ba (g) Pd (h) Kr. Periodic Table: 2 3 4 10 6 3 7 11...
-
Refer to the periodic table and write the predicted electron configuration for each of the following elements. (a) Li (b) F (c) Mg (d) P (e) Ca (f) Mn (g) Ga (h) Rb. Periodic Table: 2 3 4 10 6 3 7 11...
-
Write balanced equations based on the information given. (a) Solid magnesium + oxygen gas solid magnesium oxide (b) Nitrogen monoxide gas + oxygen gas nitrogen dioxide gas (c) Gaseous ethane(C2H6)...
-
Internet Case. Locate a comprehensive annual financial report (CAFR) for a local government from some source on the Internet, perhaps the Web site of the governmental entity or the Governmental...
-
Two in-phase loudspeakers emit identical 1000 Hz sound waves along the x-axis. What distance should one speaker be placed behind the other for the sound to have an amplitude 1.5 times that of each...
-
Writing The expected value of an accountant's profit and loss analysis is 0. Explain what this means.
-
The manager of the OBrian Glass Company is planning the production of automobile windshields for the next four months. The demand for the next four months is projected to be as shown in the following...
-
On June 1. A company purchased equipment for $50,000 from IG Company, paying $20,000 in cash and giving a one-year, 9% note for the balance. What is the adjusting entries necessary at December 31,...
-
Supply a systematic name for each of the following monoatomic anions: (a) Br (b) N 3 .
-
Refer to the periodic table and write the predicted electron configuration for each of the following elements using core notation. (a) Sr (b) Ru (c) Sb (d) Cs. Periodic Table: 2 3 4 10 6 3 7 11 Li...
-
In Problems 1968, solve each equation, if possible. x + 1 x + 2x x + 4 x + x -3 x + 3x + 2
-
Description: duff owes relatives $13,000 for college loans. find the required quarterly payment into a sinking fund if duff pays off the loan in 3 years and the interest rate is 8% per year...
-
1 3 , 9 5 0 ) Repairs and Maintenance ( $ 2 , 8 5 0 ) Utilities Expense ( $ 8 8 0 ) Operating Income $ 1 0 , 2 4 2 Other Income - Gain on Sale $ 3 0 0 Interest Expense ( $ 2 5 0 ) Earnings Before...
-
Description: The company currently has outstanding a bond with a 5.5 percent coupon rate and another bond with a 3.5 percent coupon rate. The firm has been informed by its investment banker that...
-
Find the equation of line joining the points (4, -3) and (-2, 7).
-
Calculate the work of reversible expansion of 1 mole of ideal gas at 25 degree celsius from 10 L to 20 L.
-
Which of the bonds of a carbon-carbon double bond has more effective orbital-orbital overlap, the bond or the bond?
-
Subprime loans have higher loss rates than many other types of loans. Explain why lenders offer subprime loans. Describe the characteristics of the typical borrower in a subprime consumer loan.
-
Ferraro, Inc. established a stock-appreciation rights (SAR) program on January 1, 2010, which entitles executives to receive cash at the date of exercise for the difference between the market price...
-
Access the glossary (Master Glossary) to answer the following. (a) What is the definition of basic earnings per share? (b) What is dilution? (c) What is a warrant? (d) What is a grant date?
-
For how many periods must a company present EPS data?
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


