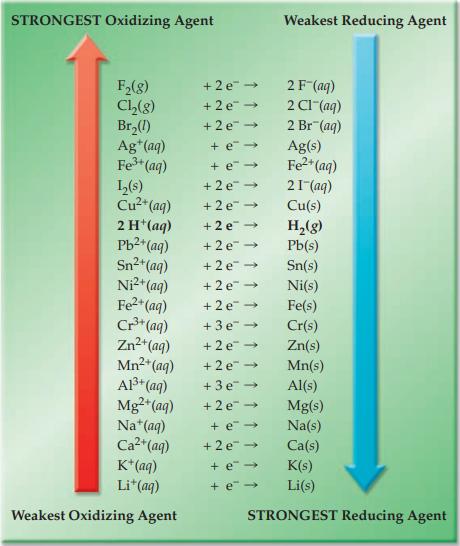
Refer to Figure 17.4 and indicate which substance in each of the following pairs has the greater
Question:
Refer to Figure 17.4 and indicate which substance in each of the following pairs has the greater tendency to be oxidized.
(a) Li(s) or K(s)
(b) Al(s) or Mg(s)
(c) Fe2+(aq) or I–(aq)
(d) Br–(aq) or Cl–(aq).
Figure 17.4
Transcribed Image Text:
STRONGEST Oxidizing Agent F₂(8) Cl₂(g) Br₂(1) Ag+ (aq) Fe³+ (aq) L₂(s) Cu²+ (aq) 2 H+ (aq) Pb²+ (aq) Sn²+(aq) Ni²+ (aq) Fe²+ (aq) Cr³+ (aq) Zn²+ (aq) Mn²+ (aq) Al³+ (aq) Mg²+ (aq) Na+ (aq) Ca²+ (aq) K+ (aq) Li+ (aq) Weakest Oxidizing Agent +2 e + 2e → +2e → + e → + e-> +2e → +2e →>> +2e → +2e → +2e → +2e → +2e → +3e → +2e → +2e → +3e → +2e → Weakest Reducing Agent + e→ +2e → + e→ + e → 2 F¯(aq) 2 Cl¯(aq) 2 Br (aq) Ag(s) Fe²+ (aq) 21-(aq) Cu(s) H₂(g) Pb(s) Sn(s) Ni(s) Fe(s) Cr(s) Zn(s) Mn(s) Al(s) Mg(s) Na(s) Ca(s) K(s) Li(s) STRONGEST Reducing Agent
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a Lis...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
According to general trends in the periodic table, predict which element in each of the following pairs has the greater metallic character. (a) K or Ca (b) Mg or Al (c) Fe or Cu (d) S or Ar. Periodic...
-
According to general trends in the periodic table, predict which element in each of the following pairs has the greater metallic character. (a) B or Al (b) Na or K (c) Mg or Ba (d) H or Fe. Periodic...
-
Refer to the periodic table and predict which element in each of the following pairs has the lower ionization energy. (a) Mg or Si (b) Pb or Bi (c) Ca or Ga (d) P or Cl. Periodic Table: 2 3 4 10 6 3...
-
At January 1, 2024, Mahmoud Industries, Inc., owed Second BancCorp $12 million under a 10% note due December 31, 2026. Interest was paid last on December 31, 2022. Mahmoud was experiencing severe...
-
Nike, Inc. reported the following plant assets and intangible assets for the year ended May 31, 2009 (in millions): other plant assets $965.8; land $221.6; patents and trademarks (at cost) $515.1;...
-
If you havent done so already, do the Try this exercises from this chapter.
-
18. Repeat the previous problem, but set = 0.05. Be sure that you simulate the riskneutral process, obtained by including the risk premium in the interest rate process.
-
The ultimate aim of governmental and not-for-prot organizations is to produce outcomes. Yet in preparing program budgets, many organizations link expenditures to outputs rather than outcomes. Why?
-
Content Area A business operated at 100% of capacity during its first month and incurred the following costs: Production costs (18,800 units): Direct materials $174,000 Direct labor 224,600 Variable...
-
Chlorine can undergo a redox reaction in which it is simultaneously oxidized and reduced. Write a balanced equation for the following in a basic solution. Cl 2 (aq) ClO 2 (aq) + Cl (aq)
-
Chlorine can undergo a redox reaction in which it is simultaneously oxidized and reduced. Write a balanced equation for the following in an acidic solution. Cl 2 (aq) Cl (aq) + HOCl(aq)
-
Derive equations for the temperature in a slab if the thermal conductivity (a) is constant, (b) varies linearly as \(k(T)=k_{0}+a\left(T-T_{0} ight)\), and (c) varies as a quadratic function...
-
6.10 Long Div. and Comp Square Calculus - No Calculator Find the indefinite integral. 1. S 4x-34x+56x-21 4x-2 dx Mastery Check #2 1 dx 2. Sx-4x+5x x-4x+5 S S Name: Sienna Nono Date: 3-1-24 Period:...
-
How well are oncology firms leveraging digital technology to gain and sustain competitive advantage?
-
Axel and Brooklyn have agreed to buy a new vehicle. Brooklyn mentions that she is looking forward to getting a new SUV, so they have room for their dogs and kids. Axel mentions he thought they were...
-
Discuss how technology and human resources are needed to operate this facility in this behind the scenes look at this retailing giant. Support your opinion with research and/or key concepts covered...
-
4.2 At a given instant, a spacecraft is 500 km above the earth, with a right ascension of 300 and a declination of -20 relative to the geocentric equatorial frame. Its velocity is 10 km/s directly...
-
Five stores in Tulsa, Oklahoma, sell the same model of a graphing calculator for $89.95, $93.49, $109.39, $93.49, and $97.69. a. What are the median price, the mean price, and the standard deviation?...
-
In Exercises 1-2, rewrite each verbal statement as an equation. Then decide whether the statement is true or false. Justify your answer. 1. The logarithm of the difference of two numbers is equal to...
-
Michelangelo Inc., a software development firm, has stock outstanding as follows: 20,000 shares of cumulative 1%, preferred stock of $25 par, and 25,000 shares of $100 par common. During its first...
-
On February 10, Peerless Rocks Inc., a marble contractor, issued for cash 40,000 shares of $10 par common stock at $34, and on May 9, it issued for cash 100,000 shares of $5 par preferred stock at...
-
On June 4, Magic Carpet Inc., a carpet wholesaler, issued for cash 250,000 shares of no-par common stock (with a stated value of $3) at $12, and on October 9, it issued for cash 25,000 shares of $75...
-
You are the digital marketing director for High West fashions, a regional clothing company that specializes in custom t-shirts. Your company has decided to launch an online advertising campaign that...
-
In-the-money put options will automatically get exercised at the expiration. True OR False
-
Which of the following examples of business-use property is NOT eligible for Section 1231 treatment when sold at a gain? * Sale of land held for three years. Net gain from a casualty gain on a dump...

Study smarter with the SolutionInn App


