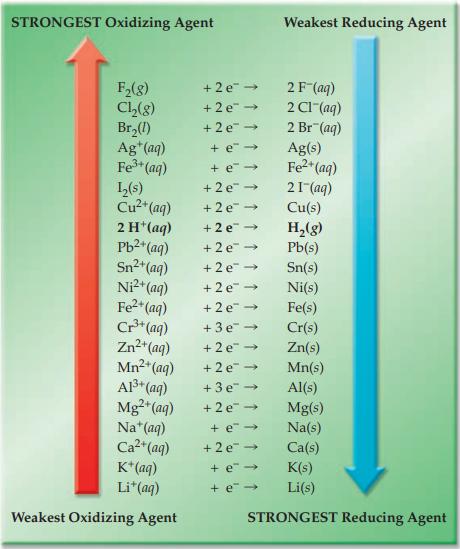
Refer to Figure 17.4 and predict which of the following metals reacts spontaneously in an aqueous FeSO
Question:
Refer to Figure 17.4 and predict which of the following metals reacts spontaneously in an aqueous FeSO4 solution.
(a) Ag
(b) Al
(c) Cr
(d) Ni.
Figure 17.4
Transcribed Image Text:
STRONGEST Oxidizing Agent F₂(g) Cl₂(g) Br₂(1) Ag+ (aq) Fe³+ (aq) 1₂(s) Cu²+ (aq) 2 H+ (aq) Pb²+ (aq) Sn²+ (aq) Ni²+ (aq) Fe²+ (aq) Cr³+ (aq) Zn²+ (aq) Mn²+ (aq) Al³+ (aq) Mg2+ (aq) Na+ (aq) Ca²+ (aq) K+ (aq) Li+ (aq) Weakest Oxidizing Agent +2 e 2 F (aq) +2e → 2 C1-(aq) +2e → 2 Br (aq) +e→ + e→ +2e → +2e → +2e → +2e → +2e → +2e → +2e → +3e → +2e → +2e → +3e → +2e → Weakest Reducing Agent +e→ +2e → + e- + e→ Ag(s) Fe²+ (aq) 21 (aq) Cu(s) H₂(g) Pb(s) Sn(s) Ni(s) Fe(s) Cr(s) Zn(s) Mn(s) Al(s) Mg(s) Na(s) Ca(s) K(s) Li(s) STRONGEST Reducing Agent
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Figure 174 lists Cr and Al as stronger reducing agents ...View the full answer

Answered By

Muhammad Ghyas Asif
It is my obligation to present efficient services to my clients by providing a work of quality, unique, competent and relevant. I hope you have confidence in me and assign me the order and i promise to follow all the instructions and keep time.
4.60+
109+ Reviews
203+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Don Jones, senior-in-charge of the audit of Stark Industries, has decided to test the following two controls for Stark's revenue process. All sales invoices are supported by proper documentation,...
-
Approximately 50% of all caregivers of older adults are caring for someone with memory loss (due to issues such as Alzheimer's or dementia). It is believed that there has been a reduction in the...
-
Refer to the periodic table and predict which of the following elements is an example of a semimetal. (a) Al (b) Ca (c) Te (d) Ti (e) Zn. 2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55 87 Fr 4 2 IIA Be 12...
-
Match the cooking or preparation method to the relevant equipment. EQUIPMENT METHOD Food processor with a grater attachment or box grater Baking Range, pot or deep fryer, spatula or tongs Boiling...
-
Draw a graph that shows roughly the shape of the probability distributions for Alta Industries, American Foam, and T-bills.
-
The drawing shows an extreme skier at the bottom of a ski jump. At this point the track is circular with a radius r. Two forces act on the skier, her weight mg and the normal force FN. Which relation...
-
What is a nonparametric test? How does a nonparametric test differ from a parametric test? What are the advantages and disadvantages of using a nonparametric test?
-
A hand-held telephone set that cost a dealer $240 less 55% and 25% is marked up 230% of cost. The dealer overhead expenses are 25% of the regular selling price. For a sales promotion, the telephone...
-
The following is a statement showing the financial status of the comapany at any given time A . Trading account B . Profit & Loss statement C . Balance Sheet D . Cash Book
-
Predict whether the following reaction is spontaneous or nonspontaneous. Ni 2+ (aq) + Sn(s) Ni(s) + Sn 2+ (aq)
-
Write a balanced ionic equation for the reaction of sodium nitrite and potassium permanganate in acidic solution. The ionic equation is MnO4 (aq) + NO (aq) Mn+ (aq) + NO3(aq)
-
1. For one week, keep a time log of your activities at work or at school. Follow the format of Figure 13.1. What does it tell you about your own time management habits?
-
From a square whose side has length \(x\), measured in inches, create a new square whose side is 5 in. longer. Find an expression for the difference between the areas of the two squares as a function...
-
Sketch the requested conic sections in Problems 14-23 using the definition. A circle with radius 5
-
Find the present value of the ordinary annuities in Problems 21-32. Amount of Deposit m 23. $250 Frequency n semiannually Rate r 8% Time t 30 yr
-
Characterize the types of investments that are most vulnerable to political risk. Characterize those that are least vulnerable. What factors influence an investments vulnerability? On a scale of 1 to...
-
Refer to the following tree diagram for a two-stage experiment. Find the probabilities in Problems 1-6. \(P(B) \) E E A B C A B C
-
The amount of merchandise that is available for sale is called supply. The amount of merchandise that consumers want to buy is called demand. Supply and demand are in equilibrium, or balance, when a...
-
Michelles trust is subject to 3.8% surtax on the lesser of the trusts net investment income or the excess of the trusts adjusted gross income over the $12,400 threshold (the highest trust tax rate)....
-
Lubricants, Inc., produces a special kind of grease that is widely used by race car drivers. The grease is produced in two processing departments: Refining and Blending. Raw materials are introduced...
-
I think we goofed when we hired that new assistant controller,? said Ruth Scarpino, president of Provost Industries. ?Just look at this report that he prepared for last month for the Finishing...
-
Gary Stevens and Mary James are production managers in the Consumer Electronics Division of General Electronics Company, which has several dozen plants scattered in locations throughout the world....
-
ABC Corporation has an activity - based costing system with three activity cost pools - Machining, Setting Up , and Other. The company's overhead costs, which consist of equipment depreciation and...
-
Consolidated Balance Sheets - USD ( $ ) $ in Thousands Dec. 3 1 , 2 0 2 3 Dec. 3 1 , 2 0 2 2 Current assets: Cash and cash equivalents $ 9 8 , 5 0 0 $ 6 3 , 7 6 9 Restricted cash 2 , 5 3 2 Short -...
-
How does corporate governance contribute to investor confidence and stakeholder trust? Accounting

Study smarter with the SolutionInn App


