Refer to the periodic table and state the highest energy sublevel for each of the following elements.
Question:
Refer to the periodic table and state the highest energy sublevel for each of the following elements.
(a) He
(b) K
(c) U
(d) Pd
(e) Be
(f) Co
(g) Si
(h) Pt.
Periodic Table:
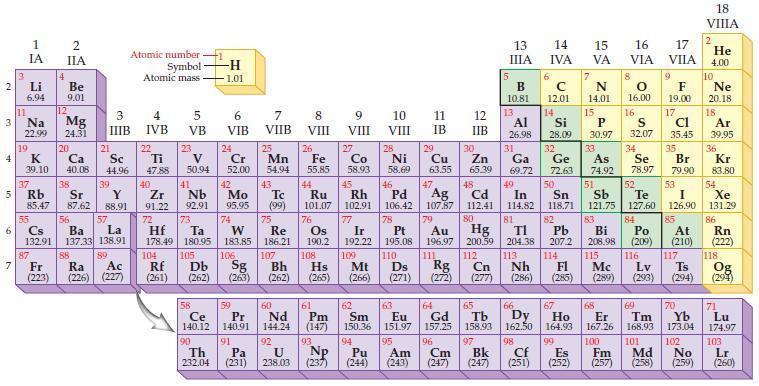
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 117 116 Mc Ts Lv (289) (293) (294) 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To determine the highest energy sublevel for each element you can look at the periodic table and ide...View the full answer

Answered By

Rishamdeep Singh
I tend to start exploring anything in science from its history or evolution, as we call it in Biology. The depth to which I have to go in explaining or helping in the completion of project is decided by the level at which my student is or the aptitude shown by him/her. The sources of information are always shared so that students are not 'spoon-fed' but take interaction with me as a learning experience. I too learn from my students and continuously try to improve on my methods.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and state the highest energy sublevel for each of the following elements. (a) H (b) Na (c) Sm (d) Br (e) Sr (f) C (g) Sn (h) Cs. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94...
-
Refer to the periodic table and state the highest energy sublevel in a silver atom. Periodic Table: 2 3 4 10 6 3 7 11 1 IA Li 6.94 Na 22.99 19 37 5 Rb K 39.10 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31...
-
Element 43 is used in medical radiology to locate tumors. Refer to the periodic table and state whether Tc has any stable isotopes. Periodic Table: 2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55 87 Fr 4 2...
-
Draw the Lewis structure of AlH 3 . Strategy Draw the Lewis structure in the normal way but realize that, in certain cases, it is not possible to place eight electrons around the central atom.
-
Activity-based Costing in a Government. The midsize City of Orangeville funds an animal control program intended to minimize the danger stray dogs pose to people and property. The program is under...
-
FIGURE EX9.20 is the force-versus-position graph for a particle moving along the x-axis. Determine the work done on the particle during each of the three intervals 01 m, 12 m, and 23 m. F, (N) 4 -x...
-
4 Tenemos dos firmas diferentes. Qu plan de remuneracin sugerira usted a cada empresa y qu razones dara para fundamentar sus recomendaciones? a) Una empresa fundada hace poco que equipo de jardinera...
-
Mukul is a teacher at Rockland School and he runs a tennis shop called Racket's Rackets. He and his wife Nikki have combined bank interest of $1,011. Nikki made $850 in tips last year. If they get a...
-
Pension data for Manu Services Inc. include the following: Discount rate Expected return on plan assets Actual return on plan assets 9% 10% Service cost, 2016 Accumulated benefit obligation 1/1/16...
-
What type of energy sublevel (s, p, d, f) is being filled by the inner transition elements?
-
What type of energy sublevel (s, p, d, f) is being filled by the transition elements?
-
Which statement is correct? A. Activity-based cost systems are less costly than traditional cost systems. B. Activity-based cost systems are easier to implement than traditional cost systems. C....
-
Pop Company holds 70% of Son Company stock. Pop has sold inventory to Son Company as follows: Percent of Sold Sales Inventory Cost to Price to Held at Year Pop Son Year end 2018 $203,000 $355,000 30%...
-
A B C D E F G H J K L 1 Cost Mortgage Payments 2 Cost Description The upscale hotel's building was acquired for $10 million, leading to monthly mortgage payments of $60,000. Behavior Dollar Amount...
-
What celebrity attributes make for effective celebrity product endorsements? Celebrity testimonials are advertising messages delivered by famous people who say or imply that they use the...
-
Initial investment of $100,000 in a new medical equipment. Interest Rate 10% (Borrowed money from a bank). Item Year 0 Year 1 Year 2 Year 3 Year 4 Year 5 Investment 100,000 Expected Cash Flow 20,000...
-
How do I draw a top view of this sketch and I was also wondering how to draw an oblique cabinet projection. + + 1 1 ' '
-
Jerome is single and cannot be claimed by anyone as a dependent. He is a student at a local university enrolled full-time in an MBA program. His tuition bill was $5,000. He paid the bill by...
-
At Glass Company, materials are added at the beginning of the process and conversion costs are added uniformly. Work in process, beginning: Number of units Transferred - in costs Direct materials...
-
(Carry back and Carry forward of NOL, No Valuation Account, No Temporary Differences) The pretax financial income (or loss) figures for Synergetic Company are as follows....
-
(Two NOLs, No Temporary Differences, No Valuation Account, Entries and Income Statement) Lanier Corporation has pretax financial income (or loss) equal to taxable income (or loss) from 2003 through...
-
(Three Differences, Classify Deferred Taxes) At December 31, 2010, Cascade Company had a net deferred tax liability of $450,000. An explanation of the items that compose this balance is as follows....
-
Management makes many judgements and estimates in preparing accounts, some of which will have a significant effect on the reported results and financial position. Give examples of ZAIN estimates and...
-
What is the NPV of a project with an initial investment of $350,000 and annual cash inflows of $150,000 for the next 10 years? Cost of capital is 13% A $436,721.21 B $442,901.59 C $452,932.43 D...
-
Journal DATE DESCRIPTION POST. REF. DEBIT CREDIT 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Joumalize the entries for the following transactions. Refer to the Chart of Accounts for exact wording of...

Study smarter with the SolutionInn App


