Refer to the periodic table and state the highest energy sublevel for each of the following elements.
Question:
Refer to the periodic table and state the highest energy sublevel for each of the following elements.
(a) H
(b) Na
(c) Sm
(d) Br
(e) Sr
(f) C
(g) Sn
(h) Cs.
Periodic Table:
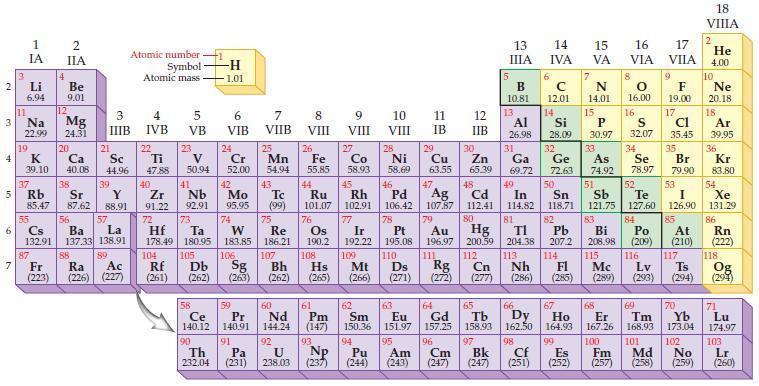
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 116 Mc Ts Lv (289) (293) (294) 117 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
To find the highest energy sublevel for each element look at the periodic table and find the row per...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and state the highest energy sublevel for each of the following elements. (a) He (b) K (c) U (d) Pd (e) Be (f) Co (g) Si (h) Pt. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94...
-
Refer to the periodic table and state the highest energy sublevel in a silver atom. Periodic Table: 2 3 4 10 6 3 7 11 1 IA Li 6.94 Na 22.99 19 37 5 Rb K 39.10 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31...
-
Refer to the periodic table and write the predicted electron configuration for each of the following elements using core notation. (a) W (b) Bi (c) Ra (d) Ac. Periodic Table: 2 3 4 10 6 3 7 11 Li...
-
Tabulate the function f(x) = sin x for x = 0.0(0.2)1.6. From this table estimate, by linear interpolation, the value of sin 1.23. Construct a table equivalent to Figure 2.102, and so estimate the...
-
Budgeting Cash Flows. Eagleview City prepares a quarterly forecast of cash flows for its water service department. Data for the upcoming quarter (in alphabetical order) measured on the cash basis are...
-
On January 1, 2017, Sunshine Ltd. acquired 30% of the outstanding ordinary shares of Moonbeam Inc. for $270,000 and now has significant influence over the investee. The fair value of Moonbeams net...
-
6 En la actualidad, un fabricante de muebles emplea representantes del fabricante para vender su lnea de muebles para sala. Estos reciben una comisin de 8%. La empresa est considerando contratar a su...
-
1. Barring illegal activities, why do you think that employees in the organizations featured in the case do not realize themselves the dangers of loosely managing proprietary and sensitive...
-
Chapter 14: The Pioneer Corporation has a target capital structure that consists of 55% debt and 45% equity. Pioneer's capital budget next year will be $10,000,000. Given that the company follows a...
-
What type of energy sublevel (s, p, d, f) is being filled by the transition elements?
-
What type of energy sublevel (s, p, d, f) is being filled by the elements in Groups IIIA/13 through VIIIA/18?
-
Gleed Company manufactures products Alpha, Beta, and Gamma from a joint process. Production, sales, and cost data for July follow. Required: 1. Assuming that joint costs are allocated using the...
-
St. Cecilia's Health System's current culture may be defined by a blend of historical ideals and problems. Given its history of primarily caring for women and children, it is likely to place a...
-
For this assignment, imagine we are the supervisor of case managers who come to us for support and guidance. You have noticed a theme of questions that are frequently asked, which mostly surround...
-
Audio Partners needs to invest in the next level of technology in order to be competitive. The company is exploring the purchase of a new piece of equipment that will cost $1,500,000, at an expected...
-
Choose a real company of their choosing and will focus on ways to help increase the company's digital consumer engagements. For example, how can the company better drive increased revenue, sales,...
-
Four morally and ethically relevant principles have been examined regarding scarcity and include: Treating people with consistency through the use of a lottery or first-come first-served basis...
-
Discuss the concept of ordinary income property and give some examples.
-
Players A, B, and C toss a fair coin in order. The first to throw a head wins. What are their respective chances of winning?
-
(Deferred Tax Asset with Previous Valuation Account) Assume the same information as E19-14, except that at the end of 2010, Callaway Corp. had a valuation account related to its deferred tax asset of...
-
(Deferred Tax Liability, Change in Tax Rate, Prepare Section of Income Statement) Sharer Inc.s only temporary difference at the beginning and end of 2010 is caused by a $2 million deferred gain for...
-
(Two Temporary Differences, Tracked through 3 Years, Multiple Rates) Taxable income and pretax financial income would be identical for Jones Co. except for its treatments of gross profit on...
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...
-
Revenue and expense items and components of other comprehensive income can be reported in the statement of shareholders' equity using: U.S. GAAP. IFRS. Both U.S. GAAP and IFRS. Neither U.S. GAAP nor...
-
Kirk and Spock formed the Enterprise Company in 2010 as equal owners. Kirk contributed land held an investment ($50,000 basis; $100,000 FMV), and Spock contributed $100,000 cash. The land was used in...

Study smarter with the SolutionInn App


