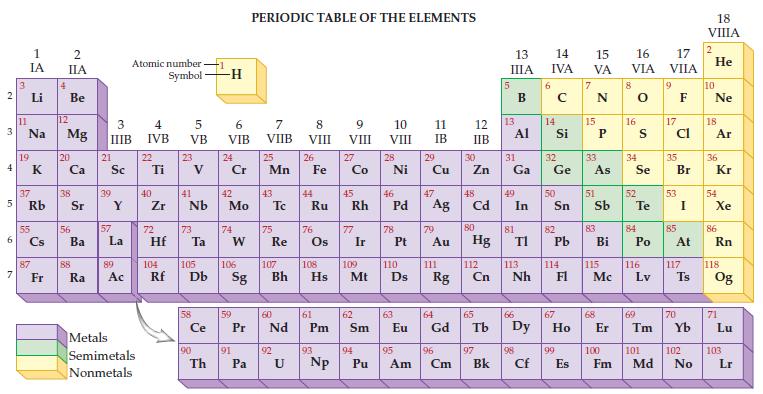
Refer to the periodic table on the inside cover of this text and determine the atomic number
Question:
Refer to the periodic table on the inside cover of this text and determine the atomic number and mass number for element 92, uranium.
Transcribed Image Text:
2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55 87 Fr 4 2 IIA Be 12 Mg 20 38 56 Ba 88 Ra 3 IIIB 21 Sc 39 57 Y La 89 Ac Atomic number Symbol Metals Semimetals Nonmetals 4 IVB 22 Ti 40 Zr 5 VB 72 23 41 Nb 73 Hf Ta Ce 90 -1 Th -H 6 VIB 24 Cr 42 Mo 74 W 59 91 PERIODIC TABLE OF THE ELEMENTS 7 VIIB Pa 25 Mn 43 75 Re 60 P Pr Nd 92 8 VIII U 26 44 Fe Ru 76 61 Pm 93 9 VIII 104 105 106 107 108 109 110 Rf Db Sg Bh Hs Mt D Np 27 Co 45 Rh 77 Ir 62 Sm 94 10 VIII Pu 28 Ni 46 78 47 Pd Ag 63 11 IB 29 95 Cu 79 Au 111 64 Eu Gd Rg 96 Am Cm 12 IIB 30 Zn 48 Cd 80 Hg 112 Cn 65 97 Bk 13 IIIA 13 31 Ga Al 49 81 In 66 TI 113 Nh 98 Cf 6 14 15 16 IVA VA VIA 14 Si 32 Ge 50 82 Pb 114 67 99 E Es 15 33 As 51 83 Bi 115 Mc 68 8 16 34 Se 52 S Te 84 Po 116 Lv 69 17 VIIA 9 17 Cl 35 53 85 Br I At 117 70 Ts 100 101 102 Fm Md No 18 VIIIA 2 He 10 Ne 18 Ar 36 Kr 54 Xe 86 Rn 118 Og 71 Lu 103 Lr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
In the periodic table we observe The atomic n...View the full answer

Answered By

BETHUEL RUTTO
Hi! I am a Journalism and Mass Communication graduate; I have written many academic essays, including argumentative essays, research papers, and literary analysis. I have also proofread and written reviews, summaries and analyses on already finished works. I am eager to continue writing!
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table on the inside cover of this text and determine the atomic number and atomic mass for iron. 2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55 87 Fr 4 2 IIA Be 12 Mg 20 38 56 Ba 88 Ra...
-
Determine the atomic number Z, the atomic mass number A, and the element X for the unknown species in the following reaction for the fission of uranium : Consult the periodic table on the inside of...
-
Refer to the periodic table on the inside front cover and indicate (a) The most nonmetallic element; (b) The transition metal with lowest atomic number; (c) A metalloid whose atomic number is exactly...
-
The following two equations have a common solution of (1, 2, 3). Which equation would complete a system of three linear equations in three variables having solution set {(1, 2, 3)}? x+y+z=6 2x = y +...
-
How do product costs affect the financial statements? How does the classification of product cost (as an asset vs. an expense) affect net income?
-
Explain how the procedure for using a valuation model to infer market expectations about a companys future growth differs from using the same model to obtain an independent estimate of value.
-
Chosen at random, 580 customers at a car dealership are contacted and asked their opinions of the service they received.
-
In 2007, Charles Riegel and his wife sued a medical device manufacturer, Medtronic. Charles had a catheter produced by Medtronic placed in his coronary artery after he suffered a heart attack....
-
full question please Homework: Chapter 11 Graded Homewo Score: 0 of 2 pts S11-11 (book/static) Thompson Turf Trimmers, Inc., reported the following financial statements for 2018: (Click the icon to...
-
Refer to the periodic table and determine the atomic number and mass number listed for element 61, promethium. 2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55 87 Fr 4 2 IIA Be 12 Mg 20 38 56 Ba 88 Ra 3 IIIB...
-
How many neutrons are in the nucleus of an atom of cobalt-60? (a) 27 (b) 33 (c) 60 (d) 87 (e) None of the above.
-
Given the pKa values of -2.2 for CH3+OH2 and 15.5 for CH3OH, calculate the pH at which (a) methanol will contain exactly equal amounts of CH3+OH2 and CH3O-; (b) 50% CH3+OH and 50% CH3+OH2 will be...
-
Companies in the tire manufacturing business use a lot of property, plant, and equipment. Tyrell Rubber and Tire Corporation and Maxwell Rubber and Tire Manufacturing are two of the leading...
-
(7%) Problem 11: A student launches a small rocket which starts from rest at ground level. At a height h = 2.09 km, the rocket reaches a speed of v = 291 m/s. At that height, the rocket runs out of...
-
2. For the following three sets of electric field lines, what charge or charges would make such lines? Indicate their locations and type of charge (e.g. positive/negative) a.
-
What is the most important take-home point that you learned from this video? https://www.youtube.com/watch?v=nUZqvsF_Wt0 2. Policy Problems. What is onepolicy that creates inequality in the labor...
-
An employee had $20,300 in gross earnings up to September 20, 2021. She has the following information for her pay for the week ending September 27, 2021. Her employer contributes 100% toward CPP and...
-
When 3-methyl-1-butene reacts with HBr, two alkyl halides are formed: 2-bromo-3 methylbutane and 2-bromo-2-methylbutane. Propose a mechanism that explains the formation of these products.
-
KD Insurance Company specializes in term life insurance contracts. Cash collection experience shows that 20 percent of billed premiums are collected in the month before they are due, 60 percent are...
-
What are holding companies? What are their advantages and disadvantages?
-
Discuss, in general, what it means for the brothers to set a credit and collections policy.
-
What is the firms expected days sales outstanding (DSO)?
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


