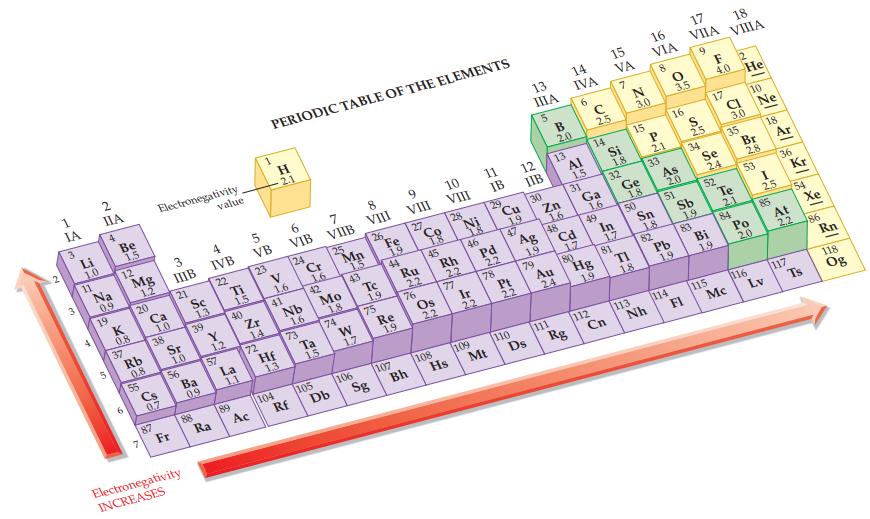
Refer to the values in Figure 12.9 and calculate the electronegativity difference in each of the following
Question:
Refer to the values in Figure 12.9 and calculate the electronegativity difference in each of the following bonds.
(a) Br—Cl
(b) Br—F
(c) I—Cl
(d) I—Br.
Figure 12.9
Transcribed Image Text:
IA 3 3 Li 1.0 11 4 IIA Bo Na 0.9 19 5 Be 1,5 12 NE K 0.8 37 Mg 1.2 20 Rb 0.8 55 Ca 1.0 Electronegativity 38 Cs 0,7 87 3 IIIB 21 Sr 1.0 56 Fr Sc 1.3 39 Ba 0.9 Electronegativity INCREASES 88 value 4 IVB 22 Y 1.2 57 Ra Ti 15 89 40 La 1.1 5 VB 23 Zr 14 72 Ac PERIODIC TABLE OF THE ELEMENTS H 2.1 V 1.6 41 Hf 13 104 6 VIB 24 Nb 16 73 Rf Cr 1.6 42 Ta 1.5 7 VIIB 105 25 Mo 1.8 74 Db Mn 1.5 43 W 1,7 8 VIII 106 26 Te 19 75 Sg Fe 22 EN ON 19 44 Re 1.9 9 VIII 107 27 Ru 22 76 Bh Co 45 Os 22 10 VIII 28 108 Rh 22 77 Hs 18 46 Ir 22 11 IB 109 29 Pd 2.2 78 Mt Cu 19 47 Pt 22 110 13 ΠΙΑ 5 12 IIB 30 Ag 1.9 79 Ds B 2.0 Zn 13 16 48 Au 111 24 14 IVA 6 Al 1.5 31 Cd 17 Rg 80 с 2.5 14 Ga 1.6 49 112 Hg 19 15 VA 7 Si 1.8 Cn 32 In 1.7 81 Ge 21 1.8. 15 50 7 TI 18. 3.0 113 16 VIA Nh P 2.1 Sn 18 8 33 82 As 20 Pb 1.9 O 3.5 51 114 17 VIIA 9 16 S 25 34 Sb 1.9 FI 83 Se 24 Bi F He 4.0 17 52 115 19 18 VIIIA CI 3.0 35 Te 2.1 Mc 84 Br 10 2.8 53 Po 20 116 Ne 18 I 25 85 3 Lv Ar 36 At Kr 117 54 2.2 Xe Ts 86 Rn 118 Og
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
a BrC130 Ax 302...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Find the kinetic energy of the a-particle emitted in the decay 28 Pu 234U+ a. The atomic masses needed are as follows: 238 Pu 234 U 238 04955 u 234 04095 u Neglect any recoil of the residual nucleus....
-
Refer to the values in Figure 12.9 and calculate the electronegativity difference in a SI bond. Figure 12.9 1 IA S Li 1.0 11 2 IIA 4 Na 0,9 19 Be 1.5 12 0.8 37 Mg 1.2 6 20 Rb 0.8 55 Ca 1.0 38 3...
-
Refer to the values in Figure 12.9 and calculate the electronegativity difference in a HP bond. Figure 12.9 1 IA S Li 1.0 11 2 IIA 4 Na 0,9 19 Be 1.5 12 0.8 37 Mg 1.2 6 20 Rb 0.8 55 Ca 1.0 38 3...
-
A job order cost accounting system is fully integrated into the general ledger of a company. Identify the major general ledger accounts used in a job order cost system. Explain how manufacturing...
-
What are the three sections of a CAFR? Briefly identify the contents of each section.
-
On January 1, 2019, Perini Company purchased an 85% interest in Silvas Company for $400,000. On this date, Silvas Company had common stock of $90,000 and retained earnings of $210,000. An examination...
-
A study found that people who suffer from moderate to severe sleep apnea are at increased risk of having high blood pressure. (Source: Journal of the American Medical Association)
-
Calculating EAR with Points you are looking at a one-year loan of $10,000. The interest rate is quoted as 9 percent plus three points. A point on a loan is simply 1 percent (one percentage point) of...
-
In making the journal entry to assign raw materials costs, a company credits Finished Goods Inventory debits Finished Goods Inventory generally credits two or more work in process accounts. often...
-
Refer to Figure 12.9 and label each atom in the following polar covalent bonds using delta notation ( + and ). (a) CH (b) SeO (c) PI (d) HBr. Figure 12.9 IA 3 3 Li 1.0 11 4 IIA Bo Na 0.9 19 5 Be...
-
Refer to the values in Figure 12.9 and calculate the electronegativity difference in each of the following bonds. (a) HCl (b) HBr (c) NO (d) CO. Figure 12.9 IA 3 3 Li 1.0 11 4 IIA Bo Na 0.9 19 5 Be...
-
Consider the linear stochastic differential equation Show that the mean E[r(t)] is governed by the following deterministic linear differential equation: while the variance var(r(t)) is governed by...
-
+ Given f(x) = x - 9 and g(x) = x+9, complete the following. (a) Find f(g(x)) and g(f(x)). (Simplify your answers completely.) f(g(x)) = g(f(x)) = (b) What does this tell us about the relationship...
-
Case Study - Rhonda Rhonda is a 28-year-old woman who has been referred to your agency by a local probation officer. Rhonda reported that she has "fired" three counselors in the past and most...
-
Calculating depreciationpartial periods LO2, 3 West Coast Tours runs boat tours along the west coast of British Columbia. On March 5, 2020, it purchased, with cash, a cruising boat for $936,000,...
-
Question 1. Write down the form of partial fractions needed to decompose the following: 482+2 (a) s32s24s 482+2 (c) s36s20 482 +2 - 4s8 (b) 8. 3 - 282 482+2 (d) s3 +2s2 - 2 Note: You are not being...
-
On December 31, 2022, Ace Hardware reported the following information on its balance sheet Accounts Receivable Allowance for Doubtful Accounts $900,000 $54,000 (credit) During 2023, the Company had...
-
Pollution control equipment must be purchased to remove the suspended organic material from liquid. being discharged from a vegetable packing plant. Two alternative pieces of equipment are available...
-
Describe basic managerial approaches to implementing controls and how these are implemented.
-
Short-tern investments why is referred stock with a dividend tied to short-tern interest rates an attractive short-term investment for corporations with excess cash?
-
Collection and disbursement floats which would a firm prefer: a net collection float or a net disbursement float? Why?
-
Float suppose a firm has a book balance of $ 2 million. At the automatic teller machine (ATM), the cash manager finds out that the bank balance is $2.5 million. What is the situation here? If this is...
-
Yard Professionals Incorporated experienced the following events in Year 1, its first year of operation: Performed services for $31,000 cash. Purchased $7,800 of supplies on account. A physical count...
-
This question is from case # 24 of book Gapenski's Cases in Healthcare Finance, Sixth Edition Select five financial and five operating Key Performance Indicators (KPIs) to be presented at future...
-
assume that we have only two following risk assets (stock 1&2) in the market. stock 1 - E(r) = 20%, std 20% stock 2- E(r) = 10%, std 20% the correlation coefficient between stock 1 and 2 is 0. and...

Study smarter with the SolutionInn App


