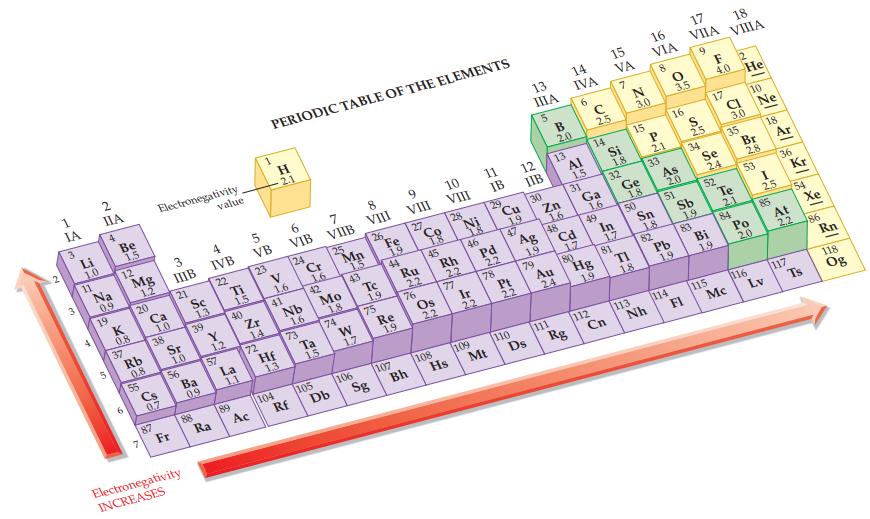
Refer to Figure 12.9 and label each atom in the following polar covalent bonds using delta notation
Question:
Refer to Figure 12.9 and label each atom in the following polar covalent bonds using delta notation (δ+ and δ–).
(a) C—H
(b) Se—O
(c) P—I
(d) H—Br.
Figure 12.9
Transcribed Image Text:
IA 3 3 Li 1.0 11 4 IIA Bo Na 0.9 19 5 Be 1,5 12 NE K 0.8 37 Mg 1.2 20 Rb 0.8 55 Ca 1.0 Electronegativity 38 Cs 0,7 87 3 IIIB 21 Sr 1.0 56 Fr Sc 1.3 39 Ba 0.9 Electronegativity INCREASES 88 value 4 IVB 22 Y 1.2 57 Ra Ti 15 89 40 La 1.1 5 VB 23 Zr 14 72 Ac PERIODIC TABLE OF THE ELEMENTS H 2.1 V 1.6 41 Hf 13 104 6 VIB 24 Nb 16 73 Rf Cr 1.6 42 Ta 1.5 7 VIIB 105 25 Mo 1.8 74 Db Mn 1.5 43 W 1,7 8 VIII 106 26 Te 19 75 Sg Fe 22 EN ON 19 44 Re 1.9 9 VIII 107 27 Ru 22 76 Bh Co 45 Os 22 10 VIII 28 108 Rh 22 77 Hs 18 46 Ir 22 11 IB 109 29 Pd 2.2 78 Mt Cu 19 47 Pt 22 110 13 ΠΙΑ 5 12 IIB 30 Ag 1.9 79 Ds B 2.0 Zn 13 16 48 Au 111 24 14 IVA 6 Al 1.5 31 Cd 17 Rg 80 с 2.5 14 Ga 1.6 49 112 Hg 19 15 VA 7 Si 1.8 Cn 32 In 1.7 81 Ge 21 1.8. 15 50 7 TI 18. 3.0 113 16 VIA Nh P 2.1 Sn 18 8 33 82 As 20 Pb 1.9 O 3.5 51 114 17 VIIA 9 16 S 25 34 Sb 1.9 FI 83 Se 24 Bi F He 4.0 17 52 115 19 18 VIIIA CI 3.0 35 Te 2.1 Mc 84 Br 10 2.8 53 Po 20 116 Ne 18 I 25 85 3 Lv Ar 36 At Kr 117 54 2.2 Xe Ts 86 Rn 118 Og
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Using delta notation the atoms in the following polar covalent bonds can be labeled as fol...View the full answer

Answered By

Surojit Das
I have vast knowledge in the field of Mathematics, Business Management and Marketing. Besides, I have been teaching on the topics Management leadership, Business Administration, Human Resource Management, Business Communication, Accounting, Auditing, Organizer Behaviours, Business Writing, Essay Writing, Copy Writing, Blog Writing since 2020. It is my personality to act quickly in any emergency situations when students need my services. I am very professional and serious in every questions students asked me at the time of dealing any projects. I have been serving detailed, quality, properly analysed research paper through the years.
4.80+
91+ Reviews
279+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to Figure 12.9 and label each atom in the following polar covalent bonds using delta notation ( + and ). (a) HS (b) OS (c) NF (d) SCl. Figure 12.9 IA 3 3 Li 1.0 11 4 IIA Bo Na 0.9 19 5 Be 1,5...
-
From the trends in the periodic table, apply delta notation and label each atom in the following polar covalent bonds: (a) NO (b) BrF.
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Reference frame S is moving along the x axis at 0.6c relative to frame S. A particle that is originally at x = 10 m at t 1 = 0 is suddenly accelerated and then moves at a constant speed of c/3 in...
-
How do operational accountability and fiscal accountability differ? In what context are they used?
-
A consolidated income statement for 2018 and comparative consolidated balance sheets for 2017 and 2018 for P Company and its 80% owned subsidiary follow: Other information: 1. Equipment depreciation...
-
A ball numbered from 1 through 52 is selected from a bin, replaced, and then a second numbered ball is selected from the bin. Classifying Events Based on Studies In Exercises 1316, identify the two...
-
Refer to the information in Problem 17-38. Suppose that Publishers, Inc., uses the FIFO method instead of the weighted-average method in all of its departments. The only changes to Problem 17-38...
-
The seven guiding principles of the Integrated Reporting Framework ( IRF ) which impact the presentation of the are as follows: - Strategic focus and future orientation - Connectivity of information...
-
Refer to Figure 12.9 and indicate which of the following are nonpolar covalent bonds. (a) ClCl (b) ClN (c) NH (d) HP. Figure 12.9 IA 3 3 Li 1.0 11 4 IIA Bo Na 0.9 19 5 Be 1,5 12 NE K 0.8 37 Mg 1.2 20...
-
Refer to the values in Figure 12.9 and calculate the electronegativity difference in each of the following bonds. (a) BrCl (b) BrF (c) ICl (d) IBr. Figure 12.9 IA 3 3 Li 1.0 11 4 IIA Bo Na 0.9 19 5...
-
The Carley Company differs from the Marple Company (described in Problem 4-21) in only one respect: it has both variable and fixed manufacturing costs. Its variable costs are $0.14 per litre and its...
-
What is the average age (measured by the variable "age") of the sample in the GSS93 subset.sav data set? Is there a significant difference in the age of those who favor the death penalty for murder...
-
Solve the system of linear equations, using the Gauss-Jordan elimination method. (If there is no solution, enter NO SOLUTION. If there are infinitely many solutions, express your answer in terms of...
-
The pay disparity is due to several reasons, one of the main ones being the old stereotypes based on the archetype of the man as the breadwinner of the family. Women are usually hired at a lower...
-
Prepare Balance Sheet: To do this activity you are required to assume the amount and line items that are to be shown on the balance sheet of your business selling homemade articles. Using the...
-
You have a "Consent to Use E-mail Communication" on file for this patient. Draft a short e-mail to her about her lab and chest X-ray results, requesting she contact the office by phone or e-mail to...
-
The City of Columbia is trying to attract a new manufacturing business to the area. It has offered to install and operate a water pumping plant to provide service to the proposed plant site. This...
-
Suppose that the electrical potential at the point (x, y, z) is E(x, y, z) = x + y - 2z. What is the direction of the acceleration at the point (1,3,2)?
-
Cash management what options are available to a firm if r believes it has too much cash? How about too little?
-
Motivations for holding cash in the chapter opening, we have discussed the enormous cash position of several companies. Why would firms such as these hold such large quantities of cash?
-
Cash management versus liquidity management what is the difference between cash management and liquidity management?
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


