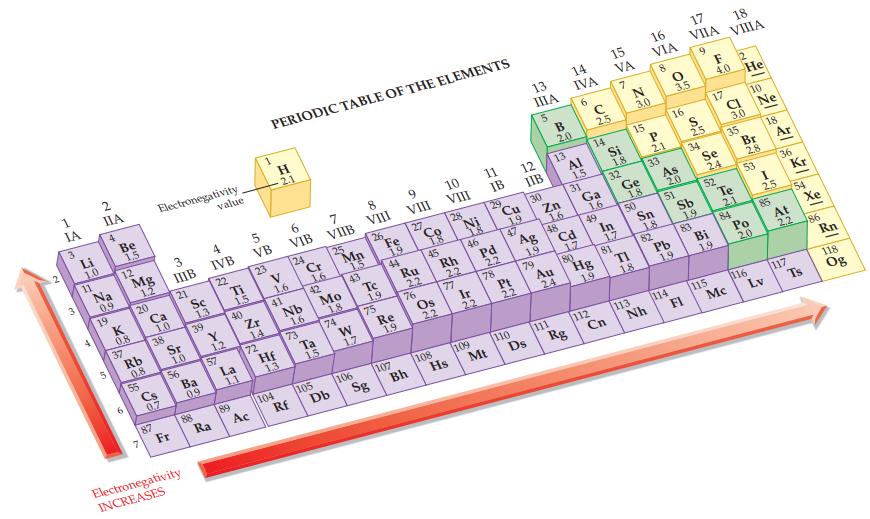
Refer to Figure 12.9 and label each atom in the following polar covalent bonds using delta notation
Question:
Refer to Figure 12.9 and label each atom in the following polar covalent bonds using delta notation (δ+ and δ–).
(a) H—S
(b) O—S
(c) N—F
(d) S—Cl.
Figure 12.9
Transcribed Image Text:
IA 3 3 Li 1.0 11 4 IIA Bo Na 0.9 19 5 Be 1,5 12 NE K 0.8 37 Mg 1.2 20 Rb 0.8 55 Ca 1.0 Electronegativity 38 Cs 0,7 87 3 IIIB 21 Sr 1.0 56 Fr Sc 1.3 39 Ba 0.9 Electronegativity INCREASES 88 value 4 IVB 22 Y 1.2 57 Ra Ti 15 89 40 La 1.1 5 VB 23 Zr 14 72 Ac PERIODIC TABLE OF THE ELEMENTS H 2.1 V 1.6 41 Hf 13 104 6 VIB 24 Nb 16 73 Rf Cr 1.6 42 Ta 1.5 7 VIIB 105 25 Mo 1.8 74 Db Mn 1.5 43 W 1,7 8 VIII 106 26 Te 19 75 Sg Fe 22 EN ON 19 44 Re 1.9 9 VIII 107 27 Ru 22 76 Bh Co 45 Os 22 10 VIII 28 108 Rh 22 77 Hs 18 46 Ir 22 11 IB 109 29 Pd 2.2 78 Mt Cu 19 47 Pt 22 110 13 ΠΙΑ 5 12 IIB 30 Ag 1.9 79 Ds B 2.0 Zn 13 16 48 Au 111 24 14 IVA 6 Al 1.5 31 Cd 17 Rg 80 с 2.5 14 Ga 1.6 49 112 Hg 19 15 VA 7 Si 1.8 Cn 32 In 1.7 81 Ge 21 1.8. 15 50 7 TI 18. 3.0 113 16 VIA Nh P 2.1 Sn 18 8 33 82 As 20 Pb 1.9 O 3.5 51 114 17 VIIA 9 16 S 25 34 Sb 1.9 FI 83 Se 24 Bi F He 4.0 17 52 115 19 18 VIIIA CI 3.0 35 Te 2.1 Mc 84 Br 10 2.8 53 Po 20 116 Ne 18 I 25 85 3 Lv Ar 36 At Kr 117 54 2.2 Xe Ts 86 Rn 118 Og
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
In polar covalent bonds the atom with higher electronegativity attracts the shared electron...View the full answer

Answered By

YOGENDRA NAILWAL
As I'm a Ph.D. student, so I'm more focussed on my chemistry laboratory. I have qualified two national level exams viz, GATE, and NET JRF (Rank 68). So I'm highly qualified in chemistry subject. Also, I have two years of teaching experience in this subject, which includes college teacher as well as a personal tutor. I can assure you if you hire me on this particular subject, you are never going to regret it.
Best Regards.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to Figure 12.9 and label each atom in the following polar covalent bonds using delta notation ( + and ). (a) CH (b) SeO (c) PI (d) HBr. Figure 12.9 IA 3 3 Li 1.0 11 4 IIA Bo Na 0.9 19 5 Be...
-
From the trends in the periodic table, apply delta notation and label each atom in the following polar covalent bonds: (a) NO (b) BrF.
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
You are a financial adviser advising the owner of a multi-national business who is considering which of two countries is the most tax-efficient in which to domicile. You are given that the owner...
-
1. Which of the following items has the greatest GAAP authority under SAS 69? a. GASB implementation guides b. Consensus positions of GASBs Emerging Issues Task Force c. GASB statements and...
-
Pillow Company purchased 90% of the common stock of Satin Company on May 1, 2016, for a cash payment of $474,000. December 31, trial balances for Pillow and Satin were: Satin Company declared a...
-
Rolling a six-sided die and then rolling the die a second time so that the sum of the two rolls is five
-
Morland Company started year 1 with $75,000 in its cash and common stock accounts. During year 1, Morland paid $50,000 cash for employee compensation and $17,500 cash for materials. Required a....
-
Raphael Corporation's balance sheet shows the following stockholders' equity section. $ 80,000 Preferred stock-5% cumulative, $ par value, 1,000 shares authorized, issued, and outstanding Common...
-
Refer to Figure 12.9 and indicate which of the following are nonpolar covalent bonds. (a) ClCl (b) ClN (c) NH (d) HP. Figure 12.9 IA 3 3 Li 1.0 11 4 IIA Bo Na 0.9 19 5 Be 1,5 12 NE K 0.8 37 Mg 1.2 20...
-
Refer to the values in Figure 12.9 and calculate the electronegativity difference in each of the following bonds. (a) BrCl (b) BrF (c) ICl (d) IBr. Figure 12.9 IA 3 3 Li 1.0 11 4 IIA Bo Na 0.9 19 5...
-
Refer to Figure 16.48. Given: = 30, = 15.5 kN/m 3 , ' = 20, and c' = 15 kN/m 2 . Find the height, H, which will have a factor of safety (F s ) of 2 against sliding along the rocksoil interface. H...
-
Read the buret (burette) volume and report your reading with the proper number of digits. Number 3.2 mL mL 0 10 15 46 20 25 30 35 47 40 48 Incorrect.
-
TCP Congestion Control using Wireshark and testmy.net. Identify the IP Address, Protocol (UDP or TCP), Destination and Source IP Address, and IP Class Type (A-D).
-
Charley & Waldo's World of Wonder is a science-oriented children's museum. The museum has a "free" section where children have unlimited use science oriented exhibits and a premium section where...
-
Let f(x) In(x). Solve each of the following equations exactly for a. (f(x)) = 11 b. f(x) = 11 c. f(x) = 11
-
Suppose the annual rate of inflation in Taiwan is 6.66%, and the annual rate of inflation in Mexico is 5.99%. If the Mexican peso depreciates relative to the Taiwan dollar by 4% in real terms, then...
-
Sam purchased a home for $150,000 with some creative financing. The bank, which agreed to lend Sam $120,000 for 6 years at 15% interest, took a first mortgage on the house. The Joneses, who sold Sam...
-
Federated Shipping, a competing overnight delivery service, informs the customer in Problem 65 that they would ship the 5-pound package for $29.95 and the 20-pound package for $59.20. (A) If...
-
Costs of Borrowing in exchange for a $400 million fixed commitment line of credit your firm has agreed to do the following. 1. Pay 1.6 percent per quarter on any funds actually borrowed. 2. Maintain...
-
Costs of Borrowing Come and Go Bank Offers your firm a 9 percent discount interest loan for up to $15 million, and in addition requires you to maintain a 5 percent compensating balance against the...
-
Cash management is it possible for a firm to have too much cash? Why would shareholders care if a firm accumulates large amount of cash?
-
Management makes many judgements and estimates in preparing accounts, some of which will have a significant effect on the reported results and financial position. Give examples of ZAIN estimates and...
-
What is the NPV of a project with an initial investment of $350,000 and annual cash inflows of $150,000 for the next 10 years? Cost of capital is 13% A $436,721.21 B $442,901.59 C $452,932.43 D...
-
Journal DATE DESCRIPTION POST. REF. DEBIT CREDIT 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Joumalize the entries for the following transactions. Refer to the Chart of Accounts for exact wording of...

Study smarter with the SolutionInn App


