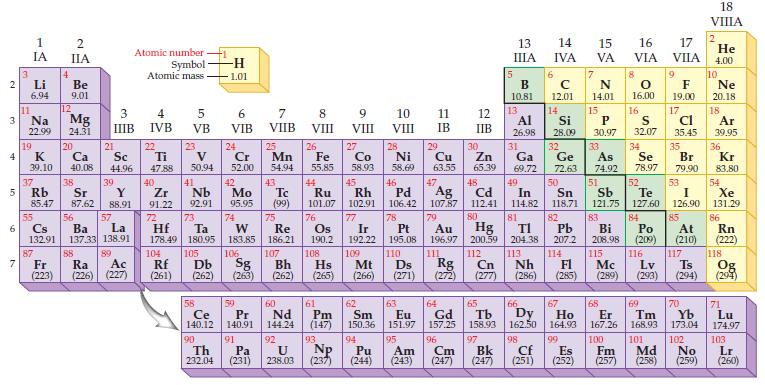
Select the symbol of the element that fits each of the following descriptions: (a) The alkali metal
Question:
Select the symbol of the element that fits each of the following descriptions:
(a) The alkali metal in the fourth period
(b) The halogen in the third period
(c) The rare earth with the lowest atomic mass
(d) The metal in Group VIIB/7 and Period 4.
Periodic Table:
Transcribed Image Text:
N 4 15 6 Be 6.94 9.01 12 11 3 Na Mg 22.99 24.31 7 3 Li 19 1 IA K 39.10 37 55 4 2 IIA 87 Fr (223) 20 21 Sc 40.08 44.96 Ca 38 Rb Sr Y 85.47 87.62 88.91 56 Cs La Ba 132.91 137.33 138.91 39 88 3 IIIB 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 104 5 VB Rf (261) 23 V 50.94 41 73 105 42 Nb Mo 92.91 95.95 58 -H Ce 140.12 1.01 90 6 VIB 72 Ta W Re Hf 178.49 180.95 183.85 186.21 Th 232.04 24 Cr Mn 52.00 54.94 74 106 7 VIIB 25 59 Pr 140.91 91 Db Sg Bh (262) (263) (262) Pa (231) 43 Tc (99) 75 107 60 Nd 144.24 92 8 VIII 26 Fe 55.85 44 Ru 101.07 76 Os 190.2 108 61 Pm (147) 93 9 VIII U NP 238.03 (237) 27 Co 58.93 45 Rh 102.91 77 Ir 192.22 109 16 VIA 8 6 9 с 12.01 14 14.01 15 O F 16.00 19.00 16 17 13 29 30 Al Si P S Cl 26.98 28.09 30.97 32.07 35.45 31 32 33 34 35 Cu Zn Ga Ge As Se Br 63.55 65.39 69.72 72.63 74.92 78.97 79.90 48 51 53 Pd Ag Cd Sb Te I 106.42 107.87 112.41 114.82 118.71 121.75 127.60 126.90 46 47 52 49 In 50 Sn 94 10 VIII Pu (244) 28 78 80 81 82 83 84 85 Pt 195.08 110 Ds Hg TI Pb Bi Po At 200.59 204.38 207.2 208.98 (209) (210) 113 Nh 112 Cn Rg 114 Fl Hs Mt (265) (266) (271) (272) (277) (286) (285) Ni 58.69 62 63 64 Sm Eu Gd 150.36 151.97 157.25 95 Am 11 IB 79 Au 196.97 111 (243) 96 13 IIIA 12 IIB Cm (247) 5 14 15 IVA VA B 10.81 66 67 164.93 65 Tb Dy Ho 158.93 162.50 97 98 Bk Cf (247) (251) 99 17 VIIA 7 Es (252) 117 115 116 Mc Lv Ts (289) (293) (294) 68 69 70 Er Tm Yb 167.26 168.93 173.04 100 101 102 Fm Md (257) (258) No (259) 18 VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 Lr (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
Referring to the periodic table in Figure 53 we have a K b Cl c Sc d ...View the full answer

Answered By

Rodrigo Louie Rey
I started tutoring in college and have been doing it for about eight years now. I enjoy it because I love to help others learn and expand their understanding of the world. I thoroughly enjoy the "ah-ha" moments that my students have. Interests I enjoy hiking, kayaking, and spending time with my family and friends. Ideal Study Location I prefer to tutor in a quiet place so that my students can focus on what they are learning.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and select the symbol of the element that fits each of the following descriptions. (a) The fourthperiod alkali metal (b) The fourthperiod alkaline earth metal (c) The rare...
-
Refer to the periodic table and select the symbol of the element that fits each of the following descriptions. (a) The thirdperiod alkali metal (b) The thirdperiod alkaline earth metal (c) The rare...
-
Refer to the periodic table and select the symbol of the element that fits each of the following descriptions. (a) The semimetal in the third period (b) The semimetal in the fourth period and Group...
-
Research the Fisher-Yates shuffling algorithm online, then use it to reimplement the shuffle method in Fig.7.12. Fig.7.12 I // Fig. 7.12: DeckOfCards.java 2 // DeckOfCards class represents a deck of...
-
Gift Shop UBIT. A local exempt organization that trains at-risk youth for employment has an annual operating budget of $300,000, which includes revenue from operating a gift shop in a nearby hotel...
-
Wastewater containing dissolved hydrogen sulfide (H 2 S) at concentration of 2.50 gmole/m 3 (85 mg/L) enters an open tank at a volumetric flow rate of 20 m 3 /h, and exits at the same volumetric flow...
-
Your marketing plan needs a market-product grid to (a) focus your marketing efforts and (b) help you create a forecast of sales for the company. Use these steps: 1 Define the market segments (the...
-
Supermart Food Stores (SFS) has experienced net operating losses in its frozen food products line in the last few periods. Management believes that the store can improve its profitability if SFS...
-
Please help me finish this problem I am so very confused!! %x) E23-17 (similar to) Question Help Top managers of Stenback Industries predicted 2018 sales of 15,100 units of its product at a unit...
-
The modern periodic law states that the properties of the elements repeat when the periodic table is arranged according to which of the following? (a) Increasing atomic number (b) Increasing mass...
-
Why did Mendeleev not include neon in his periodic table of 1871? Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 Cs 132.91 87 4 Fr (223) 2 IIA Be 9.01 12 Mg 24.31...
-
Suppose we pick a number at random from the phone book and look at the last digit. (a) What is the set of outcomes and what probability should be assigned to each outcome? (b) Would this model be...
-
Q10: Region ( experienced compressive stresses and has a than the rest of the bracket. Region ( ) experienced tension stresses and has a of the bracket. Deep Drawing and Stretch Forming width (into...
-
A sample of 1500 computer chips revealed that 32% of the chips do not fail in the first 1000 hours of their use. The company\'s promotional literature claimed that above 29% do not fail in the first...
-
The 75 lb block is released from rest 5 ft above the plate. Determine the compression of each spring when the block momentarily comes to rest after striking the plate. Neglect the mass of the plate....
-
Indiana Soy Products (OSP) buys soybeans and processes them into other soy products. Each ton of soybeans that OSP purchases for $250 can be converted for an additional $180 into 675 lbs of soy meal...
-
The 2025 Annual Report of Splish International contains the following informatio (in millions) June 29, 2025 June 27, 2024 Total assets $1,545 $1,502 Total liabilities 989 1,060 Net sales 2,800 2.971...
-
A variety of products-chicken wings, drumsticks, thighs, and so on-are the result of a joint production process of butchering a chicken that costs $0.25 per pound. The wings can be sold at the...
-
What is the maximum volume of 0.25 M sodium hypochlorite solution (NaOCl, laundry bleach) that can be prepared by dilution of 1.00 L of 0.80 M NaOCl?
-
What is the fair value option? Where do companies that elect the fair value option report unrealized holding gains and losses?
-
Moon Hardware is planning to factor some of its receivables. The cash received will be used to pay for inventory purchases. The factor has indicated that it will require recourse on the sold...
-
Kraft Enterprises owns the following assets at December 31, 2010. What amount should be reported ascash? Cash in bank-savings account Checking account balance Postdated checks Certificates of deposit...
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


