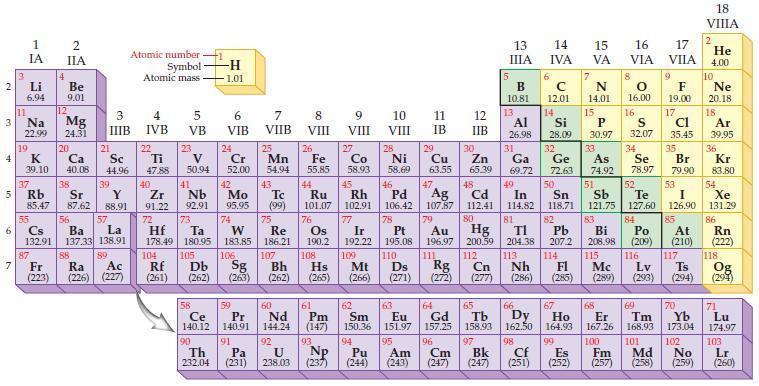
State the number of valence electrons in each of the following groups as predicted from the periodic
Question:
State the number of valence electrons in each of the following groups as predicted from the periodic table.
(a) Group IA/1
(b) Group IIIA/13
(c) Group VA/15
(d) Group VIIA/17.
Periodic Table:
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 116 Mc Ts Lv (289) (293) (294) 117 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
Explanation a Group I A contain alkali metals Lithium Sodium P...View the full answer

Answered By

Simon kingori
I am a tier-one market researcher and content developer who has been in this field for the last six years. I’ve run the freelancing gamut; from market research, data mining and SEO/SMM to copywriting, Content Development, you name it, I’ve done it. I’m extremely motivated, organized and disciplined – you have to be to work from home. My experience in Freelancing is invaluable- but what makes me a cut above the rest is my passion to deliver quality results to all my clients- it’s important to note, I've never had a dissatisfied client. Backed by a Masters degree in Computer Science from MOI university, I have the required skill set and burning passion and desire to deliver the best results for my clients. This is the reason why I am a cut above the rest. Having taken a Bsc. in computer science and statistics, I deal with all round fields in the IT category. It is a field i enjoy working in as it is dynamic and new things present themselves every day for research and exploration.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
State the number of valence electrons in each of the following groups as predicted from the periodic table. (a) Group IIA/2 (b) Group IVA/14 (c) Group VIA/16 (d) Group VIIIA/18. Periodic Table: 2 3 4...
-
Refer to the periodic table and state the number of valence electrons for any element in each of the following groups: (a) Group IIA (b) Group VA (c) Group 14 (d) Group 17. Periodic Table: 2 3 4 15...
-
Refer to the periodic table and predict the number of valence electrons for an atom of each of the following representative elements: (a) Na (b) Al (c) S (d) Xe. Periodic Table: 2 3 4 5 N.. 3 7 Li...
-
A posthole digger (the digger) is an agricultural implement manufactured by Alamo/SMC Corporation (SMC) designed, as its name implies, to dig holes in the ground for posts. The digger is...
-
Interact CaseFASB. Go to the Financial Accounting Standards Boards Web site at ww-sfasb.org. List by number and name all FASB statements that specifically provide accounting and reporting guidance...
-
Compact fluorescent bulbs are much more efficient at producing light than are ordinary incandescent bulbs. They initially cost much more, but they last far longer and use much less electricity....
-
The mean annual salary of employees at a company is $36,000 with a variance of 15,202,201.At the end of the year, each employee receives a $2000 bonus and a 4% raise (based on salary).What is the...
-
Use the data in problem 8 to create a Pareto diagram. Problem 8 In an apparel factory, every time a sewing machine breaks, the symptom is recorded. In the past 30 days, all of the sewing machine...
-
my work WS What orrect for the work you have completed so far. HH Auto Repair reports the following information for the coming year. $ 43 per labor hour 10,700 hours $2,070,000 Labor rate, including...
-
Refer to the periodic table and predict which element in each of the following pairs has the lower ionization energy. (a) Mg or Si (b) Pb or Bi (c) Ca or Ga (d) P or Cl. Periodic Table: 2 3 4 10 6 3...
-
Which group of elements has the highest ionization energy?
-
Kimbrells Furniture Co. sold a new television set and tape player to Charlie ONeil and his wife. Each purchase was on credit, and in each instance, a security agreement was executed. Later on the...
-
Part 2 One Stop Electrical Shop are merchandisers of household fixtures & fittings. The business began the last quarter of 2020 (October to December) with 25 Starburst Wall Clocks at a total cost of...
-
A species of butterfly has three subspecies A, B, and C. A scientist is trying to classify observed. specimens into these subspecies based on the color of their wings, which can be blue, green, pink,...
-
Stock during the year were sold for $8 per share. On December 31 , Portland had no remaining treasury stock. Required: Prepare the necessary journal entries to record any transactions associated with...
-
2) 20 pts. A 2-kg block rests on a wedge that has a coefficient of friction between the wedge and block of 0.3. The system accelerated to the right. Determine the maximum acceleration of the system...
-
ABC Ltd. is concerning about its poor performance and considering whether or not dropping the production and sells of product R, which incurs losses of Birr 4000. Additional information: The salaries...
-
When a passive activity is sold or otherwise disposed of, what happens to any suspended losses from that activity?
-
Trade credit from suppliers is a very costly source of funds when discounts are lost. Explain why many firms rely on this source of funds to finance their temporary working capital.
-
(EPS with Complex Capital Structure) Amy Dyken, controller at Fitzgerald Pharmaceutical Industries, a public company, is currently preparing the calculation for basic and diluted earnings per share...
-
(Basic EPS: Two-Year Presentation) Melton Corporation is preparing the comparative financial statements for the annual report to its shareholders for fiscal years ended May 31, 2010, and May 31,...
-
(Computation of Basic and Diluted EPS) Charles Austin of the controllers office of Thompson Corporation was given the assignment of determining the basic and diluted earnings per share values for the...
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


