The compound titanium oxide occurs in the mineral rutile. Use the periodic table to predict the formula
Question:
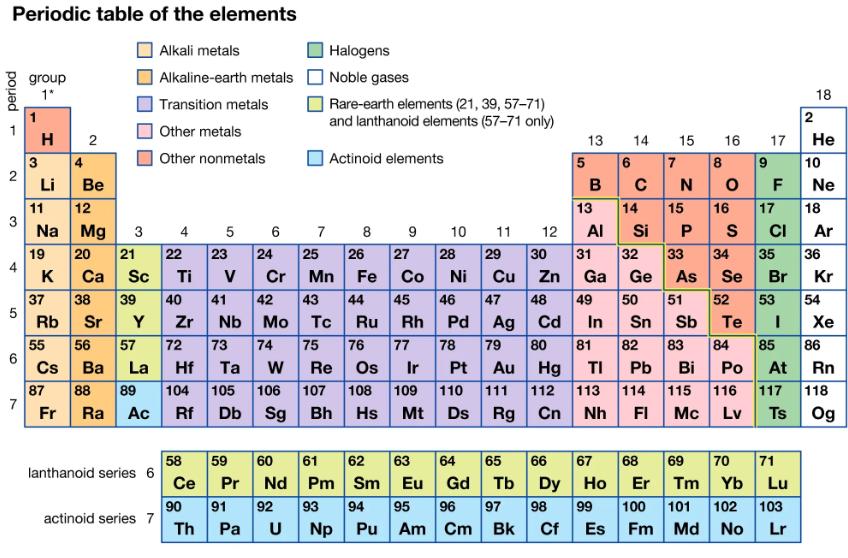
The compound titanium oxide occurs in the mineral rutile. Use the periodic table to predict the formula for titanium oxide, given the formula of zirconium oxide, ZrO2.
Transcribed Image Text:
Periodic table of the elements Alkali metals Alkaline-earth metals Transition metals Other metals Other nonmetals period - 2 3 4 5 6 7 group 1* 3 H 2 4 Li 11 Na 19 K 37 Rb 55 Cs 87 Fr Be 12 Mg 20 21 22 23 Ca Sc Ti V 38 39 40 41 Sr Y Zr 56 57 72 73 74 Ba La Hf Ta W 88 89 104 105 Ra Ac Rf 3 lanthanoid series 6 actinoid series 7 4 58 Ce 90 Th 5 es Db Sg 7 Halogens Noble gases Pa U Rare-earth elements (21, 39, 57-71) and lanthanoid elements (57-71 only) Actinoid elements 8 9 27 6 24 25 26 28 Cr Mn Fe Co Ni 45 47 48 Ag Cd Sn Sb 79 80 81 82 83 42 43 44 46 Nb Mo Tc Ru Rh Pd 75 76 77 78 Re Os Ir Pt Au Hg TI Pb Bi 107 108 109 110 111 112 113 114 Bh Hs Mt Ds Rg Cn Nh FI 59 60 61 62 63 Pr Nd Pm Sm Eu 91 92 93 94 95 Np Pu Am 10 64 Gd 96 Cm 11 29 30 Cu Zn 12 97 Bk 13 5 6 B 14 65 66 67 Tb C 49 14 31 32 Ga Ge 50 7 13 15 Al Si P 33 34 35 As Se Br 68 15 Dy Ho Er 98 99 100 Cf Es Fm N 16 17 8 OF 16 S 84 9 10 18 2 Po At 115 116 117 Mc Lv Ts He 17 18 CI 69 70 71 Tm Yb Lu 101 102 103 Md No Lr Ne Ar 51 52 53 54 Te I 85 86 36 Kr Xe Rn 118 Og
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
Titanium and zirconium are both transition metals in the same group group 4 on the ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
The compound zirconium silicate occurs in cubic zirconia. Use the periodic table to predict the formula for zirconium silicate, given the formula of titanium silicate, TiSiO 4 . Periodic table of the...
-
The element actinium is unstable and radioactive. Use the periodic table to predict the formula for actinium chloride, given the formula of lanthanum chloride, LaCl 3 . Periodic table of the elements...
-
1. Explain how an accretionary wedge forms. 2. Explain why Africa and South America are moving apart. 3. What is a hot spot? Explain how this forms a volcanic island chain such as that of the...
-
Calculate the standard entropy change for the following reactions at 25C. Comment on the sign of r S. (a) 2 Al(s) + 3 Cl 2 (g) 2 AlCl 3 (s) (b) 2 CH 3 OH() + 3 O 2 (g) 2 CO 2 (g) + 4 H 2 O(g)
-
Recording Encumbrances. During July 2010, the first month of the 2011 fiscal year, the City of Marion issued the following purchase orders and contracts (see Problem 34): General...
-
An elaborate pulley consists of four identical balls at the ends of spokes extending out from a rotating drum (Fig. Q9.18). A box is connected to a light, thin rope wound around the rim of the drum....
-
The length of time student-athletes a rock group practice each week
-
The income statements of Evans Company and Falcon Company for the current year are shown below: The following amounts were taken from the statement of changes in equity for the two companies: Evans...
-
! Required information (The following information applies to the questions displayed below.) Trey has two dependents, his daughters, ages 14 and 18, at year-end. Trey files a joint return with his...
-
Supply a systematic name for each of the following binary ionic compounds: (a) Mn 3 P 2 (b) Fe 2 S 3 .
-
Classify each of the following as a binary ionic compound, ternary ionic compound, binary molecular compound, binary acid, or ternary oxyacid: (a) Carbon disulfide, CS 2 (b) Lithium dichromate, Li 2...
-
The Group IIA carbonates decompose when heated. For example, MgCO3(s) MgO(s) + CO2(g) Use enthalpies of formation (see Appendix C) and calculate the heat required to decompose 10.0 g of magnesium...
-
2. (40 marks) Solve for y(t) such that y" - 6y' + 15y = 2 sin(3t),
-
6. Determine output class A{ ); } public static void main(String args[]) { int x; x = 10; if (x == 10) { int y = 20; System.out.print ("x and y: y = x*2; + y); } y = 100; } System.out.print ("x and...
-
Anita and Bonita have been roommates for the past two years while they've been in graduate school. Now that they're graduating, they are each planning to move to different cities. Their one joint...
-
To what extent are business ethics assumed, or taken for granted, by people in businesses?
-
Empowered by what he has learned in this class about gender, Brady makes a friendly wager with his girfriend, Marlisa: "I bet I can guess how the men and women at the next table will behave during...
-
a. At what pH will 99% of a compound with a pKa of 8.4 be in its basic form? b. At what pH will 91% of a compound with a pKa of 3.7 be in its acidic form? c. At what pH will 9% of a compound with a...
-
A consumer magazine is evaluating five brands of trash compactors for their effectiveness in reducing the volume of typical household products that are discarded. In the experiment, each block...
-
On January 5, 2010, Phelps Corporation received a charter granting the right to issue 5,000 shares of $100 par value, 8% cumulative and nonparticipating preferred stock, and 50,000 shares of $10 par...
-
(Treasury Stock Transactions and Presentation) Clemson Company had the following stockholders equity as of January 1, 2010. Common stock, $5 par value, 20,000 shares issued $100,000 Paid-in capital...
-
Hatch Company has two classes of capital stock outstanding: 8%, $20 par preferred and $5 par common. At December 31, 2010, the following accounts were included in stockholders equity. Preferred...
-
Show that the convexity for a zero coupon bond with m payments per year is (m) n(n + -)(1+ m m
-
Abdul Canarte , a Central Bank economist, noticed that the total group purchasing basket of goods (CPI) has gone from $149,740.00 to $344,460.00 in 8 years. With monthly compounding, what is the...
-
ABC Corporation expects sales next year to be $50,000,000. Inventory and accounts receivable (combined) will increase $8,000,000 to accommodate this sales level. The company has a profit margin of 6...

Study smarter with the SolutionInn App


