The element actinium is unstable and radioactive. Use the periodic table to predict the formula for actinium
Question:
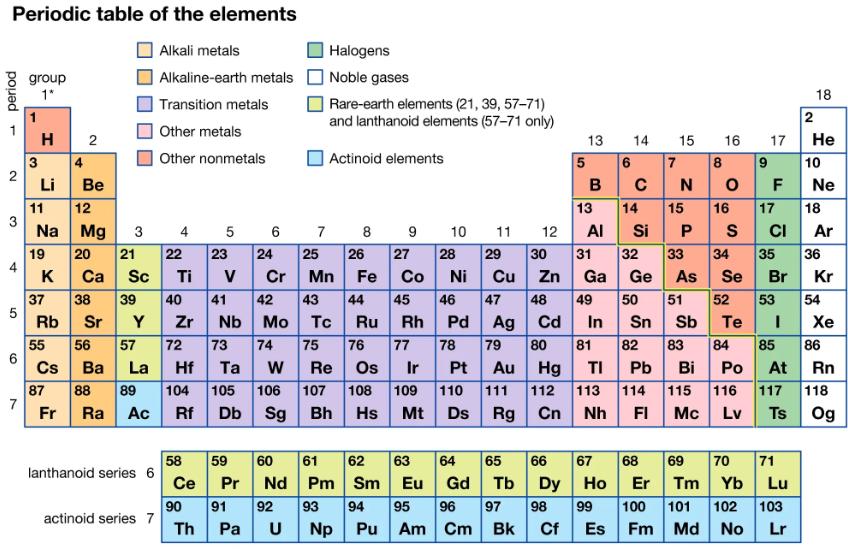
The element actinium is unstable and radioactive. Use the periodic table to predict the formula for actinium chloride, given the formula of lanthanum chloride, LaCl3.
Transcribed Image Text:
Periodic table of the elements Alkali metals Alkaline-earth metals Transition metals Other metals Other nonmetals period - 2 3 4 5 6 7 group 1* 3 H 2 4 Li 11 Na 19 K 37 Rb 55 Cs 87 Fr Be 12 Mg 20 21 22 23 Ca Sc Ti V 38 39 40 41 Sr Y Zr 56 57 72 73 74 Ba La Hf Ta W 88 89 104 105 Ra Ac Rf 3 lanthanoid series 6 actinoid series 7 4 58 Ce 90 Th 5 es Db Sg 7 Halogens Noble gases Pa U Rare-earth elements (21, 39, 57-71) and lanthanoid elements (57-71 only) Actinoid elements 8 9 27 6 24 25 26 28 Cr Mn Fe Co Ni 45 47 48 Ag Cd Sn Sb 79 80 81 82 83 42 43 44 46 Nb Mo Tc Ru Rh Pd 75 76 77 78 Re Os Ir Pt Au Hg TI Pb Bi 107 108 109 110 111 112 113 114 Bh Hs Mt Ds Rg Cn Nh FI 59 60 61 62 63 Pr Nd Pm Sm Eu 91 92 93 94 95 Np Pu Am 10 64 Gd 96 Cm 11 29 30 Cu Zn 12 97 Bk 13 5 6 B 14 65 66 67 Tb C 49 14 31 32 Ga Ge 50 7 13 15 Al Si P 33 34 35 As Se Br 68 15 Dy Ho Er 98 99 100 Cf Es Fm N 16 17 8 OF 16 S 84 9 10 18 2 Po At 115 116 117 Mc Lv Ts He 17 18 CI 69 70 71 Tm Yb Lu 101 102 103 Md No Lr Ne Ar 51 52 53 54 Te I 85 86 36 Kr Xe Rn 118 Og
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The element actinium Ac falls under the actinide series of the periodic table which are found below ...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
The element lawrencium is unstable and radioactive. Use the periodic table to predict the chemical formula for lawrencium chloride, given the formula of lutetium chloride, LuCl 3 .
-
The compound titanium oxide occurs in the mineral rutile. Use the periodic table to predict the formula for titanium oxide, given the formula of zirconium oxide, ZrO 2 . Periodic table of the...
-
The compound zirconium silicate occurs in cubic zirconia. Use the periodic table to predict the formula for zirconium silicate, given the formula of titanium silicate, TiSiO 4 . Periodic table of the...
-
Differentiate f(x) = log 10 (2 + sin x).
-
Explain the essential differences between extraordinary items and special items and how each of these items should be reported on the government-wide statement of activities.
-
What is the major difference between a CMO and the other types of mortgage-related securities?
-
Blood Donations A survey asked a sample of people how many times they donate blood each year. The random variable x represents the number of donations in one year. Use the histogram to find the...
-
Part b of Example 7 used the central limit theorem to approximate the probability of coming out ahead if you bet $10 on red on each of 40 different roulette wheel spins. For each spin, the winnings...
-
(!) Consider an economy that can be either in a boom or in a recession with equal\ probabilities each year. Two investment funds generate the following annual returns\ depending on the state of the...
-
Write the chemical formula for the following binary compounds given their constituent ions: (a) Iron(II) sulfide, Fe 2+ and S 2- (b) Mercury(I) fluoride, Hg 2 2 + and F - (c) Lead(IV) oxide, Pb 4+...
-
Classify each of the following as binary ionic, ternary ionic, binary molecular, binary acid, or ternary oxyacid: NaCl, HCl, HCl(aq), NaClO 3 , HClO 3 (aq).
-
Fill in the blank to indicate the line item that would appear on the income statement for each of the following events. Item on Income Statement Event ____________________ Disposed of a segment of...
-
You have two dashboards in the same workspace named Production and Manufacturing. Your company's Power BI administrator creates the following two dashboard data classifications: Medium Impact (MEDI)...
-
Question 2: Red Rocks Corporation was organized on September 1. Red Rocks encountered the following events during the first month of operations. a. Received $65,000 cash from the investors who...
-
he previous three weeks of data is below for the sales of sheds at SHEDS INC. Calculate the forecast for the next perioud (week 4) using a two period weighted moving average using weights of 3 and 2....
-
/3 3) ST tan(x) - In(cosx) dx What is the value of u? us dulcis) What is the corresponding value of du? du= 1-5mx dx cosx You must show all of your work in the space below to earn full credit. 9/3 So...
-
Please use the file which provides the data to answer the problems 1-3. Problem 1) The time Students entered the classroom of OM 390, Introductory Operations Management, was recorded by the professor...
-
a. For each of the acid-base reactions in Section 1.17, compare the pKa values of the acids on either side of the equilibrium arrows and convince yourself that the position of equilibrium is in the...
-
Fahrad Inc. sells all of its product on account. Fahrad has the following accounts receivable payment experience: Percent paid in the month of sale .........10 Percent paid in the month after the...
-
Over what period of time should compensation cost be allocated?
-
How compensation expense is computed using the fair value approach?
-
What are the advantages of using restricted stock to compensate employees?
-
please help Problem 13-7 (Algo) Prepare a Statement of Cash Flows [LO13-1, LO13-2] [The following information applies to the questions displayed below.] Comparative financial statements for Weaver...
-
A firm has 1000 shareholders, each of whom own $59 in shares. The firm uses $28000 to repurchase shares. What percentage of the firm did each of the remaining shareholders own before the repurchase,...
-
Vancouver Bank agrees to lend $ 180,000 to Surrey Corp. on November 1, 2020 and the company signs a six-month, 6% note maturing on May 1, 2021. Surrey Corp. follows IFRS and has a December 31 fiscal...

Study smarter with the SolutionInn App


