The compound zirconium silicate occurs in cubic zirconia. Use the periodic table to predict the formula for
Question:
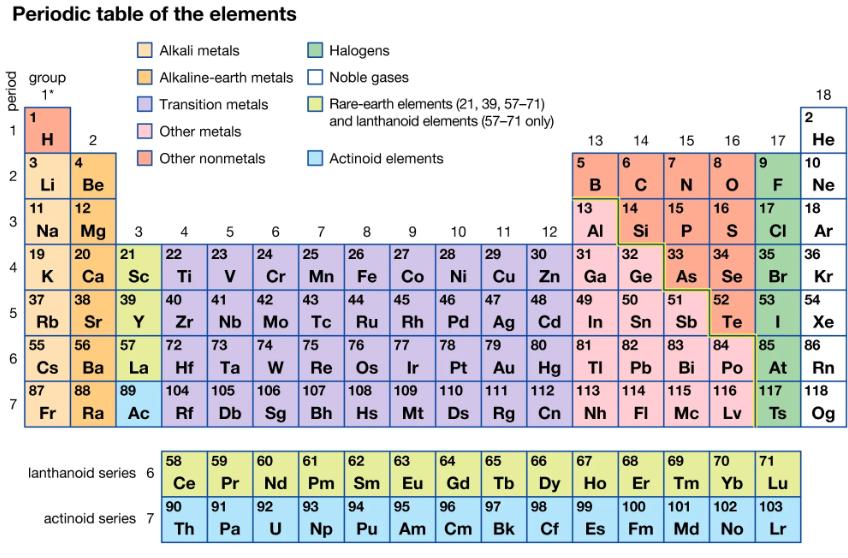
The compound zirconium silicate occurs in cubic zirconia. Use the periodic table to predict the formula for zirconium silicate, given the formula of titanium silicate, TiSiO4.
Transcribed Image Text:
Periodic table of the elements Alkali metals Alkaline-earth metals Transition metals Other metals Other nonmetals period - 2 3 4 5 6 7 group 1* 3 H 2 4 Li 11 Na 19 K 37 Rb 55 Cs 87 Fr Be 12 Mg 20 21 22 23 Ca Sc Ti V 38 39 40 41 Sr Y Zr 56 57 72 73 74 Ba La Hf Ta W 88 89 104 105 Ra Ac Rf 3 lanthanoid series 6 actinoid series 7 4 58 Ce 90 Th 5 es Db Sg 7 Halogens Noble gases Pa U Rare-earth elements (21, 39, 57-71) and lanthanoid elements (57-71 only) Actinoid elements 8 9 6 24 25 26 28 Cr Mn Fe Co Ni 45 47 48 Ag Cd Sn Sb 79 80 81 82 83 42 43 44 46 Nb Mo Tc Ru Rh Pd 75 76 77 78 Re Os Ir Pt Au Hg TI Pb Bi 107 108 109 110 111 112 113 114 Bh Hs Mt Ds Rg Cn Nh FI 27 59 60 61 62 63 Pr Nd Pm Sm Eu 91 92 93 94 95 Np Pu Am 10 64 Gd 96 Cm 11 29 30 Cu Zn 12 97 Bk 13 5 6 B 14 65 66 67 Tb C 49 14 31 32 Ga Ge 50 7 13 15 Al Si P 33 34 35 As Se Br 68 15 Dy Ho Er 98 99 100 Cf Es Fm N 16 17 8 OF 16 S 84 9 10 18 2 Po At 115 116 117 Mc Lv Ts He 17 18 CI 69 70 71 Tm Yb Lu 101 102 103 Md No Lr Ne Ar 51 52 53 54 Te I 85 86 36 Kr Xe Rn 118 Og
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
Formula for zirconium silicate ZrSiO can be predicted based on the formula for titanium silicate TiS...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
The compound titanium oxide occurs in the mineral rutile. Use the periodic table to predict the formula for titanium oxide, given the formula of zirconium oxide, ZrO 2 . Periodic table of the...
-
The element actinium is unstable and radioactive. Use the periodic table to predict the formula for actinium chloride, given the formula of lanthanum chloride, LaCl 3 . Periodic table of the elements...
-
The element lawrencium is unstable and radioactive. Use the periodic table to predict the chemical formula for lawrencium chloride, given the formula of lutetium chloride, LuCl 3 .
-
A sealed flask contains water and oxygen gas at 25C. The O 2 gas has a partial pressure of 1.5 atm. (a) What is the concentration of O 2 in the water? (b) If the pressure of O 2 in the flask is...
-
Recording Adopted Budget. The City of Marion adopted the following General Fund budget for fiscal year 2011: Estimated revenues: Taxes................$3,000,000 Intergovernmental revenues...........
-
You make two versions of the same object out of the same material having uniform density. For one version, all the dimensions are exactly twice as great as for the other one. If the same torque acts...
-
The distance a baseball travels
-
Gladmark Company produces two types of get-well cards: scented and regular. Drivers for the four activities are as follows: The following activity data have been collected: Inspecting products...
-
Question 4: Axe Inc. recorded the following transactions for the just-completed month: a. $40,000 in raw materials was purchased on account. b. $115,000 in raw materials was requisitioned for use in...
-
Supply a systematic name for each of the following binary ionic compounds: (a) Mn 3 P 2 (b) Fe 2 S 3 .
-
Classify each of the following as a binary ionic compound, ternary ionic compound, binary molecular compound, binary acid, or ternary oxyacid: (a) Carbon disulfide, CS 2 (b) Lithium dichromate, Li 2...
-
Find any horizontal or vertical asymptotes. f(x) = 6x-x-2 2x+x-6
-
In Exercises 29 and 30, find the probabilities and indicate when the "5% guideline for cumbersome calculations" is used. 29. Medical Helicopters In a study of helicopter usage and patient survival,...
-
Introduction to Internetworking Project 1: Ctrl-Alt-Del Inc. INTRODUCTION You have accepted a contract to participate in the design of the network infrastructure of a company called Ctrl-Alt-Del Inc....
-
Construct Arguments Tell whether each statement is always true, sometimes true, or never true. Explain. a. An integer is a whole number. b. A natural number is a rational number. c. An irrational...
-
Please answer the following Questions : 1. Who are the competitors for Whole Foods? 2. Do you consider traditional supermarkets to be competitors for natural and organic supermarkets? 3. How would...
-
LNC Corp is trying to determine the effect of its inventory turnover ratio and DSO on its cash conversion. Credit sales in 2016 is $101,000, cost of goods sold will be 70% of sales and it earned a...
-
Draw resonance contributors for the following compounds: a. b. O: T:O o: O: :O O:
-
Chao, Louis, and Mari, unrelated individuals, own all of the shares of Cerise Corporation. All three shareholders have been active in the management of Cerise since its inception. In the current...
-
Seles Corporations charter authorized issuance of 100,000 shares of $10 par value common stock and 50,000 shares of $50 preferred stock. The following transactions involving the issuance of shares of...
-
Before Gordon Corporation engages in the treasury stock transactions listed below, its general ledger reflects, among others, the following account balances (par value of its stock is $30 per share)....
-
(Treasury StockCost MethodEquity Section Preparation) Washington Company has the following stockholders equity accounts at December 31, 2010. Common Stock$100 par value, authorized 8,000...
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Brief Exercise 10-6 Flint Inc. purchased land, building, and equipment from Laguna Corporation for a cash payment of $327,600. The estimated fair values of the assets are land $62,400, building...
-
"faithful respresentation" is the overriding principle that should be followed in ones prepaparation of IFRS-based financial statement. what is it? explain it fully quoting IAS. how this this...

Study smarter with the SolutionInn App


