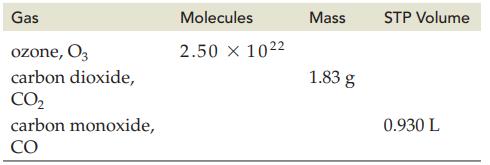
Use the given quantity for each gas listed to complete the following table. Gas ozone, 03 carbon
Question:
Use the given quantity for each gas listed to complete the following table.
Transcribed Image Text:
Gas ozone, 03 carbon dioxide, CO₂ carbon monoxide, CO Molecules 2.50 × 1022 Mass 1.83 g STP Volume 0.930 L
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
The formula I used to calculated the ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Use the given quantity for each gas listed to complete the following table. Gas methane, CH ethane, CH6 propane, C3Hg Molecules 1.50 x 1023 Mass 7.50 g STP Volume 5.58 L
-
Start of Payroll Project 7-3a October 9, 20-- No. 1 The first payroll in October covered the two workweeks that ended on September 26 and October 3. This payroll transaction has been entered for you...
-
The first payroll in October covered the two workweeks that ended on September 26 and October 3. This payroll transaction has been entered for you in the payroll register, the employees' earnings...
-
Reisen Travel offers helicopter service from suburban towns to John F. Kennedy International Airport in New York City. Each of its 10 helicopters makes between 1,000 and 2,000 round-trips per year....
-
Erik Co. elects to use the percentage-of-sales basis in 2012 to record bad debts expense. It estimates that 2% of net credit sales will become uncollectible. Sales revenues are $800,000 for 2012,...
-
Two depository institutions have composite CAMELS ratings of 1 or 2 and are well capitalized. Thus, each institution falls into the FDIC Risk Category I deposit insurance assessment scheme....
-
E8.2. Calculating ROCE from the Statement of Shareholders' Equity (Easy) From the following information, calculate the return on common equity for the year 2009 (amounts in millions of dollars)....
-
Describe the process of subdivision of activities and events that composes the tree diagram known as the Work Breakdown Structure or Gozinto chart. Why is the input of responsible managers and...
-
A call with a strike price of $50 costs $3. A put with the same strike price and expiration date costs $2. Lets consider an option portfolio which consists of buying 1 call and buying 2 puts. The...
-
If the percent sodium in a salt crystal, NaCl, is 39.34%, what is the percent sodium in a kilogram of salt?
-
Calculate the volume in liters for each of the following gases at STP. (a) 5.05 g of nitrogen, N 2 (b) 4.18 x 10 24 molecules of ethane, C 2 H 6 .
-
How much heat must be removed from 1.96 kg of water at 0 C to make ice cubes at 0 C?
-
What is brand awareness for Jam & Daisies ? their leaning advantage, consideration advantage, choice advantages? 5. what is the recommendation of brand awareness? 6. What is Brand recognition? 7....
-
On August 1st, Custom Car Co's work in process inventory was $24900; its raw materials inventory was $6000; manufacturing overhead had a $1800 debit balance. Work in Process Subsidiary Data 8/1:...
-
Case: Castoro & Partners, CPAs is auditing Cloud 9 for the FY2023. Cloud 9 is a small public company and has been an audit client of Castoro & Partners since 2018. Materiality Methodology: Overall...
-
1)Solve the following differential equations by Undetermined Coefficient Method. dy dx dy - 4- 4+ 4y = 16x2e2x dx
-
Every year Monty Industries manufactures 8,600 units of part 231 for use in its production cycle. The per unit costs of part 231 are as follows: Direct materials Direct labor Variable manufacturing...
-
(a) Verify that at high frequencies Wien's law is a good approximation to Planck's blackbody equation. (b) In June 1900 Rayleigh applied the equipartition theorem of classical statistical mechanics...
-
For all of the following words, if you move the first letter to the end of the word, and then spell the result backwards, you will get the original word: banana dresser grammar potato revive uneven...
-
What are the components of an individuals attitude?
-
Think of a person that you know who seems to have positive affectivity. Think of another who has more negative affectivity. How constant are they in their expressions of mood and attitude?
-
How does perception affect behavior?
-
You are evaluating a new project for the firm you work for, a publicly listed firm. The firm typically finances new projects using the same mix of financing as in its capital structure, but this...
-
state, "The subscription price during a rights offering is normally r; lower ; lower r; higher er; higher than the rights-on price and
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...

Study smarter with the SolutionInn App


