An nth-order chemical reaction with one reactant obeys the differential equation dc -kc, dt ||
Question:
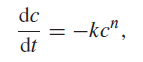
where c is the concentration of the reactant and k is a constant. Solve this differential equation by separation of variables. If the initial concentration is c0 moles per liter, find an expression for the time required for half of the reactant to react.
Transcribed Image Text:
dc -kc", dt ||
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (15 reviews)
For half of the original amo...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Mathematics questions
-
A reactant in a first-order chemical reaction without back reaction has a concentration governed by the same formula as radioactive decay, where [A] 0 is the concentration at time t = 0, [A] t is the...
-
A reactant in a first-order chemical reaction without back reaction has a concentration governed by the same formula as radioactive decay, where [A] 0 is the concentration at time t = 0,[A] t is the...
-
In a second-order chemical reaction involving one reactant and having no back reaction, Solve this differential equation by separation of variables. Do a definite integration from t = 0 to t = t 1 ....
-
Nisha has completed her MBA and has joined a company which was going to raise fund from long term sources such as Debt and Equity. Nisha was asked by her manager to prepare a report on which could be...
-
Joan's Grocery Store made the following Form 941 payroll tax deposits during the look-back period of July 1, 201A, through June 30, 201B: Quarter Ended .................................. Amount Paid...
-
This problem continues the Daniels Consulting situation from Problem P14-46 of Chapter 14. Assuming Daniels Consultings net income for the year was $90,537 and knowing that the current market price...
-
In studying the product life cycle in the commercial mainframe computer market over the period 1968 to 1982, Shane Greenstein (Northwestern University) and James Wade (University of Illinois) found...
-
A telemarketing firm has studied the effects of two factors on the response to its television advertisements. The first factor is the time of day at which the ad is run, while the second is the...
-
ASAP
-
Imagine that Howard has asked you to write some queries to help him make better use of his data. For each information request below, write a single query that provides the answer set. When a task...
-
If z c (t) is a general solution to the complementary equation and zp(t) is a particular solution to the inhomogeneous equation, show that z c + z p is a solution to the inhomogeneous equation of Eq....
-
Locate the time at which z attains its maximum value and find the maximum value.
-
With its tradition of a job for life for most citizens, Japan once had a much lower unemployment rate than that of Canada; from 1960 to 1995, the unemployment rate in Japan exceeded 3% only once....
-
A parent acquires all of the stock of a subsidiary for $40 million in cash. The subsidiarys books report the following account balances at the date of acquisition (in trial balance format)....
-
1. Given: The sign for the Inn of the Prancing Pony in Bree-yes, it comes in pints-is fixed on the end of a beam of length 5L. If the sigh deflects too much then Gandalf will hit his head when he...
-
Q21) Add positive and negative charges as shown in the diagram below. Use the arrows of the simulation to guide you in drawing continuous electric field lines around and in between the three charges....
-
When 10.1 g CaO is dropped into a styrofoam coffee cup containing 157 g H2O at 18.0C, the temperature rises to 35.8C. Calculate the enthalpy change of the following reaction in kJ/mol CaO. Assume...
-
4-12. Sometimes heterogeneous chemical reactions take place at the walls of tubes in which reactive mixtures are flowing. If species A is being consumed at a tube wall because of a chemical reaction,...
-
let R[x] have the ordering given by i. P low ii. P High as described in Example 25.2. In each case (i) and (ii), list the labels a, b, c, d, e of the given polynomials in an order corresponding to...
-
State whether each of the following will increase or decrease the power of a one-way between-subjects ANOVA. (a) The effect size increases. (b) Mean square error decreases. (c) Mean square between...
-
If we focus on patients where there are different results for the supine and upright images [i.e., groups (iii) and (iv) above], what test can be performed to assess whether there is a significant...
-
Perform the test in Problem 10.17 and report a p-value (two-tailed). Interpret the results in words? Cardiology, Radiology The conventions of cardiac echocardiography are derived from comprehensive...
-
Perform the test in Problem 10.17 and report a p-value (two-tailed). Interpret the results in words? Cardiology, Radiology The conventions of cardiac echocardiography are derived from comprehensive...
-
You borrowed $15,000 for buying a new car from a bank at an interest rate of 12% compounded monthly. This loan will be repaid in 48 equal monthly installments over four years. Immediately after the...
-
Discuss how debt restructuring, settlement, or modification works. Discuss the journal entries for debtor and creditor
-
Could CNL be a viable business? If so, under what conditions and what level of production (and, since production is directly related to production workers, employees)? All information provided for...

Study smarter with the SolutionInn App


